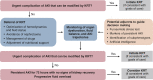
Indications for and Timing of Initiation of KRT
- PMID: 36100262
- PMCID: PMC10101614
- DOI: 10.2215/CJN.05450522
Indications for and Timing of Initiation of KRT
Abstract
KRT is considered for patients with severe AKI and associated complications. The exact indications for initiating KRT have been debated for decades. There is a general consensus that KRT should be considered in patients with AKI and medically refractory complications ("urgent indications"). "Relative indications" are more common but defined with less precision. In this review, we summarize the latest evidence from recent landmark clinical trials, discuss strategies to anticipate the need for KRT in individual patients, and propose an algorithm for decision making. We emphasize that the decision to consider KRT should be made in conjunction with other forms of organ support therapies and important nonkidney factors, including the patient's preferences and overall goals of care. We also suggest future research to differentiate patients who benefit from timely initiation of KRT from those with imminent recovery of kidney function. Until then, efforts are needed to optimize the initiation and delivery of KRT in routine clinical practice, to minimize nonessential variation, and to ensure that patients with persistent AKI or progressive organ failure affected by AKI receive KRT in a timely manner.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
S.M. Bagshaw is supported by a Canada Research Chair in Critical Care Outcomes and Systems Evaluation; reports consultancy agreements with Baxter, BioPorto, and Novartis; reports research funding from Baxter; reports honoraria from Baxter; reports fees from Baxter for scientific advisory and speaking; reports fees from BioPorto for scientific advisory and clinical adjudication; reports fees from Novartis for scientific advisory; serves as an associate editor for
Figures
References
-
- Ostermann M Bellomo R Burdmann EA Doi K Endre ZH Goldstein SL Kane-Gill SL Liu KD Prowle JR Shaw AD Srisawat N Cheung M Jadoul M Winkelmayer WC Kellum JA; Conference Participants : Controversies in acute kidney injury: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int 98: 294–309, 2020 - PMC - PubMed
-
- Bagshaw SM, Darmon M, Ostermann M, Finkelstein FO, Wald R, Tolwani AJ, Goldstein SL, Gattas DJ, Uchino S, Hoste EA, Gaudry S: Current state of the art for renal replacement therapy in critically ill patients with acute kidney injury. Intensive Care Med 43: 841–854, 2017 - PubMed
-
- Ostermann M, Dickie H, Barrett NA: Renal replacement therapy in critically ill patients with acute kidney injury--When to start. Nephrol Dial Transplant 27: 2242–2248, 2012 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials