Increasing anti-S antibody testing: a quality improvement initiative with evolving COVID-19 guidelines
- PMID: 36100293
- PMCID: PMC9471206
- DOI: 10.1136/bmjoq-2022-001886
Increasing anti-S antibody testing: a quality improvement initiative with evolving COVID-19 guidelines
Abstract
Background: COVID-19 management guidelines are constantly evolving, making them difficult to implement practically. Ronapreve was a neutralising monoclonal antibody introduced into UK COVID-19 guidelines in 2021. It reduces mortality in seronegative patients infected with non-omicron variants. Antibody testing on admission is therefore vital in ensuring patients could be considered for Ronapreve as inpatients.
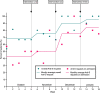
Local problem: We found that on our COVID-19 ward, 31.4% of patients were not having anti-S tests despite fulfilling the other criteria to be eligible for Ronapreve. This was identified as an important target to improve; by not requesting anti-S tests, we were forgoing the opportunity to use an intervention that could improve outcomes.
Methods: We analysed patient records for patients with COVID-19 admitted to our ward over 4 months to observe if awareness of the need to request anti-S increased through conducting plan-do-study-act (PDSA) cycles.
Interventions: Our first intervention was an multidisciplinary team (MDT) discussion at our departmental audit meeting highlighting our baseline findings and the importance of anti-S requesting. Our second intervention was to hang printed posters in both the doctors' room and the ward as a visual reminder to staff. Our final intervention was trust-wide communications of updated local COVID-19 guidance that included instructions for anti-S requesting on admission.
Results: Our baseline data showed that only 68.6% of patients with symptomatic COVID-19 were having anti-S antibody tests requested. This increased to 95.0% following our three interventions. There was also a reduction in the amount of anti-S requests being 'added on', from 57.1% to 15.8%.
Conclusions: COVID-19 guidelines are constantly evolving and require interventions that can be quickly and easily implemented to improve adherence. Sustained reminders through different approaches allowed a continued increase in requesting. This agrees with research that suggests a mixture of educational sessions and visual reminders of guidelines increase their application in clinical practice.
Keywords: COVID-19; Quality improvement; Quality measurement.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- United Kingdom Government . Patients admitted to hospital. Available: https://coronavirus.data.gov.uk/details/healthcare?areaType=overview&are... [Accessed 21 Feb 2022].
-
- Randomised evaluation of COVID-19 therapy. results. Available: https://www.recoverytrial.net/results [Accessed 21 Feb 2022].
-
- Medicines & Healthcare products Regulatory Agency . Summary of product characteristics for Ronapreve. Available: https://www.gov.uk/government/publications/regulatory-approval-of-ronapr... [Accessed 21 Feb 2022].
-
- Department of Health & Social Care . Antibody testing for SARS-CoV-2: key information. Available: https://www.gov.uk/government/publications/antibody-testing-for-sars-cov... [Accessed 21 Feb 2022].
-
- Randomised Evaluation of COVID-19 Therapy (RECOVERY) . RECOVERY trial finds Regeneron’s monoclonal antibody combination reduces deaths for hospitalised COVID-19 patients who have not mounted their own immune response. Available: https://www.recoverytrial.net/news/recovery-trial-finds-regeneron2019s-m... [Accessed 21 Feb 2022].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical