Role of necroptosis in traumatic brain and spinal cord injuries
- PMID: 36100321
- PMCID: PMC9481937
- DOI: 10.1016/j.jare.2021.12.002
Role of necroptosis in traumatic brain and spinal cord injuries
Abstract
Background: Traumatic brain injury (TBI) and spinal cord injury (SCI) are capable of causing severe sensory, motor and autonomic nervous system dysfunctions. However, effective treatments for TBI and SCI are still unavailable, mainly because the death of nerve cells is uncontrollable. Necroptosis is a type of programmed cell death and a critical mechanism in the process of neuronal cell death. However, the role of necroptosis has not been comprehensively defined in TBI and SCI.
Aim of review: This review aimed to summarize the role of necroptosis in central nervous system (CNS) trauma and its therapeutic implications and present important suggestions for researchers conducting in-depth research.
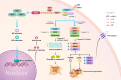
Key scientific concepts of review: Necroptosis is orchestrated by a complex comprising the receptor-interacting protein kinase (RIPK)1, RIPK3 and mixed lineage kinase domain-like protein (MLKL) proteins. Mechanistically, RIPK1 and RIPK3 form a necrosome with MLKL. After MLKL dissociates from the necrosome, it translocates to the plasma membrane to induce pore formation in the membrane and then induces necroptosis. In this review, the necroptosis signalling pathway and the execution of necroptosis are briefly discussed. In addition, we focus on the existing information on the mechanism by which necroptosis participates in CNS trauma, particularly in the temporal pattern of RIPKs and in different cell types. Furthermore, we describe the association of miRNAs and necroptosis and the relationship between different types of CNS trauma cell death. Finally, this study highlights agents likely capable of curtailing such a type of cell death according to results optimization and CNS trauma and presents important suggestions for researchers conducting in-depth research.
Keywords: CNS trauma; Cell death; Necroptosis; Spinal cord injury; Traumatic brain injury.
Copyright © 2022. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Feigin V.L., Nichols E., Alam T., Bannick M.S., Beghi E., Blake N., Culpepper W.J., Dorsey E.R., Elbaz A., Ellenbogen R.G., Fisher J.L. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–480. - PMC - PubMed
-
- Healy E., Dempsey M., Lally C., Ryan M.P. Apoptosis and necrosis: mechanisms of cell death induced by cyclosporine A in a renal proximal tubular cell line. Kidney Int. 1998;54(6):1955–1966. - PubMed
-
- Metzstein M.M., Stanfield G.M., Horvitz H.R. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 1998;14(10):410–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

