A vancomycin resistance-associated WalK(S221P) mutation attenuates the virulence of vancomycin-intermediate Staphylococcus aureus
- PMID: 36100324
- PMCID: PMC9481939
- DOI: 10.1016/j.jare.2021.11.015
A vancomycin resistance-associated WalK(S221P) mutation attenuates the virulence of vancomycin-intermediate Staphylococcus aureus
Abstract
Introduction: Vancomycin-intermediate Staphylococcus aureus (VISA) is typically associated with a decline in virulence. We previously reported a WalK(S221P) mutation that plays an important role in mediating vancomycin resistance in VISA XN108. Whether this mutation is implicated in bacterial virulence remains unknown.
Objectives: This study aimed to investigate the effect of WalK(S221P) mutation on the virulence of VISA and the underlying mechanism of this effect.
Methods: The influence of WalK(S221P) mutation on VISA virulence and its underlying mechanism were explored using animal models, RNA-seq analysis, RT-qPCR, hemolytic assay, slide coagulase test, Western blot, β-galactosidase assay, and electrophoresis mobility shift assay (EMSA).
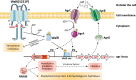
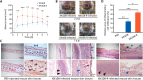
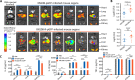
Results: Compared with XN108, WalK(S221P)-reverted strain XN108-R exacerbated cutaneous infections with increased lesion size and extensive inflammatory infiltration in mouse models. The bacterial loads of S. aureus XN108-R in murine kidney increased compared with those of XN108. RNA-seq analysis showed upregulation of a set of virulence genes in XN108-R, which exhibited greater hemolytic and stronger coagulase activities compared with XN108. Introduction of WalK(S221P) to methicillin-resistant S. aureus USA300 and methicillin-susceptible strain Newman increased the vancomycin resistance of the mutants, which exhibited reduced hemolytic activities and decreased expression levels of many virulence factors compared with their progenitors. WalK(S221P) mutation weakened agr promoter-controlled β-galactosidase activity. EMSA results showed that WalK-phosphorylated WalR could directly bind to the agr promoter region, whereas WalK(S221P)-activated WalR reduced binding to the target promoter. Inactivation of agr in S. aureus did not affect their vancomycin susceptibility but mitigated the virulence alterations caused by WalK(S221P) mutation.
Conclusion: The results of our study indicate that WalK(S221P) mutation can enhance vancomycin resistance in S. aureus of diverse genetic backgrounds. WalK(S221P)- bearing S. aureus strains exhibit reduced virulence. WalK(S221P) mutation may directly impair the activation of the agr system by WalR, thereby decreasing the expression of virulence factors in VISA.
Keywords: Agr system; Staphylococcus aureus; Vancomycin resistance; Virulence; WalK(S221P); WalKR.
Copyright © 2022. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
WalK(S221P), a naturally occurring mutation, confers vancomycin resistance in VISA strain XN108.J Antimicrob Chemother. 2017 Apr 1;72(4):1006-1013. doi: 10.1093/jac/dkw518. J Antimicrob Chemother. 2017. PMID: 27999059
-
WalK(S221P) Mutation Promotes the Production of Staphylococcus aureus Capsules Through an MgrA-Dependent Pathway.Microorganisms. 2025 Feb 25;13(3):502. doi: 10.3390/microorganisms13030502. Microorganisms. 2025. PMID: 40142395 Free PMC article.
-
A novel mutation of walK confers vancomycin-intermediate resistance in methicillin-susceptible Staphylococcus aureus.Int J Med Microbiol. 2021 Feb;311(2):151473. doi: 10.1016/j.ijmm.2021.151473. Epub 2021 Jan 6. Int J Med Microbiol. 2021. PMID: 33445057
-
Staphylococcus aureus response and adaptation to vancomycin.Adv Microb Physiol. 2024;85:201-258. doi: 10.1016/bs.ampbs.2024.04.006. Epub 2024 Jun 1. Adv Microb Physiol. 2024. PMID: 39059821 Review.
-
Mechanisms of vancomycin resistance in Staphylococcus aureus.J Clin Invest. 2014 Jul;124(7):2836-40. doi: 10.1172/JCI68834. Epub 2014 Jul 1. J Clin Invest. 2014. PMID: 24983424 Free PMC article. Review.
Cited by
-
Virulence attributes of successful methicillin-resistant Staphylococcus aureus lineages.Clin Microbiol Rev. 2023 Dec 20;36(4):e0014822. doi: 10.1128/cmr.00148-22. Epub 2023 Nov 20. Clin Microbiol Rev. 2023. PMID: 37982596 Free PMC article. Review.
-
Beta-Lactam Antibiotics Promote Extracellular Vesicle Production of Staphylococcus aureus Through ROS-Mediated Lipid Metabolic Reprogramming.J Extracell Vesicles. 2025 May;14(5):e70077. doi: 10.1002/jev2.70077. J Extracell Vesicles. 2025. PMID: 40314062 Free PMC article.
-
Genomic and phenotypic characterization of multidrug-resistant Staphylococcus haemolyticus isolated from burn patients in Chongqing, southwestern China.Microbiol Spectr. 2025 Jun 3;13(6):e0257724. doi: 10.1128/spectrum.02577-24. Epub 2025 Apr 17. Microbiol Spectr. 2025. PMID: 40243352 Free PMC article.
-
Loaded delta-hemolysin shapes the properties of Staphylococcus aureus membrane vesicles.Front Microbiol. 2023 Oct 6;14:1254367. doi: 10.3389/fmicb.2023.1254367. eCollection 2023. Front Microbiol. 2023. PMID: 37869662 Free PMC article.
-
Neobavaisoflavone Inhibits Biofilm Formation and α-Toxin Activity of Staphylococcus aureus.Curr Microbiol. 2023 Jun 26;80(8):258. doi: 10.1007/s00284-023-03355-4. Curr Microbiol. 2023. PMID: 37358668
References
-
- Das S., Lindemann C., Young B.C., Muller J., Österreich B., Ternette N., et al. Natural mutations in a Staphylococcus aureus virulence regulator attenuate cytotoxicity but permit bacteremia and abscess formation. Proc Natl Acad Sci USA. 2016;113(22):E3101–E3110. doi: 10.1073/pnas.1520255113. - DOI - PMC - PubMed
-
- Patel J.B. CLSI; Wayne, PA, USA: 2014. Performance standards for antimicrobial susceptibility testing: twenty-fourth informational supplement.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

