Practice and challenges for organ donation after medical assistance in dying: A scoping review including the results of the first international roundtable in 2021
- PMID: 36100362
- PMCID: PMC10092544
- DOI: 10.1111/ajt.17198
Practice and challenges for organ donation after medical assistance in dying: A scoping review including the results of the first international roundtable in 2021
Abstract
The procedure combining medical assistance in dying (MAiD) with donations after circulatory determination of death (DCDD) is known as organ donation after euthanasia (ODE). The first international roundtable on ODE was held during the 2021 WONCA family medicine conference as part of a scoping review. It aimed to document practice and related issues to advise patients, professionals, and policymakers, aiding the development of responsible guidelines and helping to navigate the issues. This was achieved through literature searches and national and international stakeholder meetings. Up to 2021, ODE was performed 286 times in Canada, the Netherlands, Spain, and Belgium, including eight cases of ODE from home (ODEH). MAiD was provided 17,217 times (2020) in the eight countries where ODE is permitted. As of 2021, 837 patients (up to 14% of recipients of DCDD donors) had received organs from ODE. ODE raises some important ethical concerns involving patient autonomy, the link between the request for MAiD and the request to donate organs and the increased burden placed on seriously ill MAiD patients.
Keywords: clinical research/practice; donors and donation: donation after circulatory death (DCD); guidelines; organ procurement; organ transplantation in general; patient safety; primary care; solid organ transplantation.
© 2022 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures
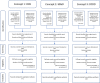

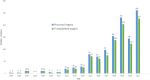
References
-
- Euthanasia Code 2018: review procedures in practice. Regional euthanasia review committees. Published online 2018. Accessed May 20, 2022. https://www.euthanasiecommissie.nl/binaries/euthanasiecommissie/document...
-
- Termination of life on request and assisted suicide act. WTL Published; 2001. Accessed May 20, 2022. https://wetten.overheid.nl/BWBR0012410/2020‐01‐01
-
- Project de loi relatif à l'euthanasie. Doc 501488/001. Chambre Des Représentants de Belgique. Published; 2002. Accessed May 20, 2022. www.worldrtd.org/BelgiumLawTransl.html
-
- Law of March 16, 2009 on palliative care, advance directive and end‐of‐life support. Journal officiel du Grand‐Duché de Luxembourg. Accessed May 20, 2022. http://legilux.public.lu/eli/etat/leg/loi/2009/03/16/n1/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

