Defining new reference intervals for serum free light chains in individuals with chronic kidney disease: Results of the iStopMM study
- PMID: 36100605
- PMCID: PMC9470548
- DOI: 10.1038/s41408-022-00732-3
Defining new reference intervals for serum free light chains in individuals with chronic kidney disease: Results of the iStopMM study
Abstract
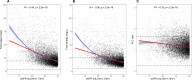
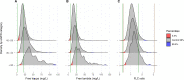
Serum free light chain (FLC) concentration is greatly affected by kidney function. Using a large prospective population-based cohort, we aimed to establish a reference interval for FLCs in persons with chronic kidney disease (CKD). A total of 75422 participants of the iStopMM study were screened with serum FLC, serum protein electrophoresis and immunofixation. Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine. Central 99% reference intervals were determined, and 95% confidence intervals calculated. Included were 6461 (12%) participants with measured FLCs, eGFR < 60 mL/min/1.73 m2, not receiving renal replacement therapy, and without evidence of monoclonality. Using current reference intervals, 60% and 21% had kappa and lambda FLC values outside the normal range. The FLC ratio was outside standard reference interval (0.26-1.65) in 9% of participants and outside current kidney reference interval (0.37-3.10) in 0.7%. New reference intervals for FLC and FLC ratio were established. New reference intervals for the FLC ratio were 0.46-2.62, 0.48-3.38, and 0.54-3.30 for eGFR 45-59, 30-44, and < 30 mL/min/1.73 m2 groups, respectively. The crude prevalence of LC-MGUS in CKD patients was 0.5%. We conclude that current reference intervals for FLC and FLC ratio are inaccurate in CKD patients and propose new eGFR based reference intervals to be implemented.
© 2022. The Author(s).
Conflict of interest statement
Dr. Landgren has received grant support from: NCI/NIH, FDA, LLS, Rising Tide Foundation, Memorial Sloan Kettering Cancer Center, MMRF, IMF, Paula, and Rodger Riney Foundation, Perelman Family Foundation, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, Karyopharm; has received honoraria for scientific talks/participated in advisory boards for: Adaptive, Amgen, Binding Site, BMS, Celgene, Cellectis, Glenmark, Janssen, Juno, Pfizer; and served on Independent Data Monitoring Committees (IDMC) for international randomized trials by: Takeda, Merck, Janssen, Theradex. Dr. Hultcrantz has recevied grant support from GSK, Amgen, Daiichi Sankyo, Cosette Pharmaceuticals, and has received honoraria for scientific talks/participated in advisory boards for BMS, Curio Science, and Intellisphere LLC. All other authors declare no potential conflicts of interest.
Figures

References
-
- Kyle RA, Durie BGM, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7. doi: 10.1038/leu.2010.60. - DOI - PMC - PubMed
-
- Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ, Colby CL, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8. doi: 10.1016/S0140-6736(10)60482-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

