Shank2/3 double knockout-based screening of cortical subregions links the retrosplenial area to the loss of social memory in autism spectrum disorders
- PMID: 36100669
- PMCID: PMC9763120
- DOI: 10.1038/s41380-022-01756-8
Shank2/3 double knockout-based screening of cortical subregions links the retrosplenial area to the loss of social memory in autism spectrum disorders
Abstract
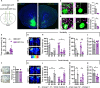
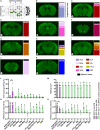
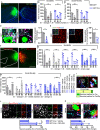
Members of the Shank protein family are master scaffolds of the postsynaptic architecture and mutations within the SHANK genes are causally associated with autism spectrum disorders (ASDs). We generated a Shank2-Shank3 double knockout mouse that is showing severe autism related core symptoms, as well as a broad spectrum of comorbidities. We exploited this animal model to identify cortical brain areas linked to specific autistic traits by locally deleting Shank2 and Shank3 simultaneously. Our screening of 10 cortical subregions revealed that a Shank2/3 deletion within the retrosplenial area severely impairs social memory, a core symptom of ASD. Notably, DREADD-mediated neuronal activation could rescue the social impairment triggered by Shank2/3 depletion. Data indicate that the retrosplenial area has to be added to the list of defined brain regions that contribute to the spectrum of behavioural alterations seen in ASDs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Differentially altered social dominance- and cooperative-like behaviors in Shank2- and Shank3-mutant mice.Mol Autism. 2020 Oct 30;11(1):87. doi: 10.1186/s13229-020-00392-9. Mol Autism. 2020. PMID: 33126897 Free PMC article.
-
Differential effectiveness of dietary zinc supplementation with autism-related behaviours in Shank2 knockout mice.Philos Trans R Soc Lond B Biol Sci. 2024 Jul 29;379(1906):20230230. doi: 10.1098/rstb.2023.0230. Epub 2024 Jun 10. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38853567 Free PMC article.
-
SHANK1 polymorphisms and SNP-SNP interactions among SHANK family: A possible cue for recognition to autism spectrum disorder in infant age.Autism Res. 2019 Mar;12(3):375-383. doi: 10.1002/aur.2065. Epub 2019 Jan 10. Autism Res. 2019. PMID: 30629339
-
Comparison of SHANK3 deficiency in animal models: phenotypes, treatment strategies, and translational implications.J Neurodev Disord. 2021 Nov 16;13(1):55. doi: 10.1186/s11689-021-09397-8. J Neurodev Disord. 2021. PMID: 34784886 Free PMC article. Review.
-
Modeling autism by SHANK gene mutations in mice.Neuron. 2013 Apr 10;78(1):8-27. doi: 10.1016/j.neuron.2013.03.016. Neuron. 2013. PMID: 23583105 Free PMC article. Review.
Cited by
-
Cell-Specific Single Viral Vector CRISPR/Cas9 Editing and Genetically Encoded Tool Delivery in the Central and Peripheral Nervous Systems.eNeuro. 2024 Jul 5;11(7):ENEURO.0438-23.2024. doi: 10.1523/ENEURO.0438-23.2024. Print 2024 Jul. eNeuro. 2024. PMID: 38871457 Free PMC article.
-
Fetal exposure to valproic acid dysregulates the expression of autism-linked genes in the developing cerebellum.Transl Psychiatry. 2023 Apr 5;13(1):114. doi: 10.1038/s41398-023-02391-9. Transl Psychiatry. 2023. PMID: 37019889 Free PMC article.
-
Rapid Eye Movements in Sleep Furnish a Unique Probe into the Ontogenetic and Phylogenetic Development of the Visual Brain: Implications for Autism Research.Brain Sci. 2025 May 26;15(6):574. doi: 10.3390/brainsci15060574. Brain Sci. 2025. PMID: 40563746 Free PMC article. Review.
-
Disrupted extracellular matrix and cell cycle genes in autism-associated Shank3 deficiency are targeted by lithium.Mol Psychiatry. 2024 Mar;29(3):704-717. doi: 10.1038/s41380-023-02362-y. Epub 2023 Dec 20. Mol Psychiatry. 2024. PMID: 38123724 Free PMC article.
-
Shank3 deletion in PV neurons is associated with abnormal behaviors and neuronal functions that are rescued by increasing GABAergic signaling.Mol Autism. 2023 Aug 1;14(1):28. doi: 10.1186/s13229-023-00557-2. Mol Autism. 2023. PMID: 37528484 Free PMC article.
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM–5). American Psychiatric Publishing: United States, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

