Manganese Porphyrin Promotes Post Cardiac Arrest Recovery in Mice and Rats
- PMID: 36101338
- PMCID: PMC9312251
- DOI: 10.3390/biology11070957
Manganese Porphyrin Promotes Post Cardiac Arrest Recovery in Mice and Rats
Abstract
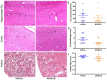
Introduction Cardiac arrest (CA) and resuscitation induces global cerebral ischemia and reperfusion, causing neurologic deficits or death. Manganese porphyrins, superoxide dismutase mimics, are reportedly able to effectively reduce ischemic injury in brain, kidney, and other tissues. This study evaluates the efficacy of a third generation lipophilic Mn porphyrin, MnTnBuOE-2-PyP5+, Mn(III) ortho meso-tetrakis (N-n-butoxyethylpyridinium-2-yl)porphyrin (MnBuOE, BMX-001), in both mouse and rat models of CA. Methods Forty-eight animals were subjected to 8 min of CA and resuscitated subsequently by chest compression and epinephrine infusion. Vehicle or MnBuOE was given immediately after resuscitation followed by daily subcutaneous injections. Body weight, spontaneous activity, neurologic deficits, rotarod performance, and neuronal death were assessed. Kidney tubular injury was assessed in CA mice. Data were collected by the investigators who were blinded to the treatment groups. Results Vehicle mice had a mortality of 20%, which was reduced by 50% by MnBuOE. All CA mice had body weight loss, spontaneous activity decline, neurologic deficits, and decreased rotarod performance that were significantly improved at three days post MnBuOE daily treatment. MnBuOE treatment reduced cortical neuronal death and kidney tubular injury in mice (p < 0.05) but not hippocampus neuronal death (23% MnBuOE vs. 34% vehicle group, p = 0.49). In rats, they had a better body-weight recovery and increased rotarod latency after MnBuOE treatment when compared to vehicle group (p < 0.01 vs. vehicle). MnBuOE-treated rats had a low percentage of hippocampus neuronal death (39% MnBuOE vs. 49% vehicle group, p = 0.21) and less tubular injury (p < 0.05) relative to vehicle group. Conclusions We demonstrated the ability of MnBuOE to improve post-CA survival, as well as functional outcomes in both mice and rats, which jointly account for the improvement not only of brain function but also of the overall wellbeing of the animals. While MnBuOE bears therapeutic potential for treating CA patients, the females and the animals with comorbidities must be further evaluated before advancing toward clinical trials.
Keywords: BMX-001; cardiac arrest; functional deficit; ischemia/reperfusion; manganese porphyrin; outcome.
Conflict of interest statement
I.B.-H., I.S., D.S.W. and Duke University have patent rights and have licensed technologies to BioMimetix J.V., L.L.C., I.B.-H., I.S. and D.S.W. are consultants with BioMimetix J.V., L.L.C. Other authors have no conflicts of interest to declare.
Figures





References
-
- Kuroki N., Abe D., Suzuki K., Mikami M., Hamabe Y., Aonuma K., Sato A. Exercise-related resuscitated out-of-hospital cardiac arrest due to presumed myocardial ischemia: Result from coronary angiography and intravascular ultrasound. Resuscitation. 2018;133:40–46. doi: 10.1016/j.resuscitation.2018.09.023. - DOI - PubMed
-
- Lavinio A., Scudellari A., Gupta A.K. Hemorrhagic shock resulting in cardiac arrest: Is therapeutic hypothermia contraindicated? Minerva Anestesiol. 2012;78:969–970. - PubMed
LinkOut - more resources
Full Text Sources

