Prevalence and Antimicrobial Susceptibility of Bovine Mycoplasma Species in Egypt
- PMID: 36101462
- PMCID: PMC9312167
- DOI: 10.3390/biology11071083
Prevalence and Antimicrobial Susceptibility of Bovine Mycoplasma Species in Egypt
Abstract
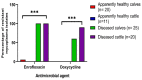
Among many bovine Mycoplasma species (spp.), Mycoplasma bovis is recognized as a significant causative agent of respiratory diseases in cattle. In recent years, resistant M. bovis isolates, especially to fluoroquinolones, have been reported globally as a result of the extensive usage of antimicrobials in the treatment of bovine pneumonia. Therefore, the aim of this study is to investigate the prevalence and antimicrobial susceptibility patterns of bovine Mycoplasma spp. isolated from the respiratory tracts of cattle in Egypt and to assess the fluoroquinolones resistance in the recovered mycoplasma isolates via broth microdilution and conventional PCR techniques. Conventional phenotypic methods identified 128 mycoplasma isolates (32%) from 400 different samples, with M. bovis being the predominant spp. (61%), followed by M. bovirhinis (15%). Of note, mycoplasma isolates were rarely isolated from total healthy lung tissues (7/55, 12.7%), but they were frequently isolated from pneumonic lungs (31/45, 68.9%). All the examined mycoplasma isolates (n = 76) were sensitive to tilmicosin, tylosin, tulathromycin, spiramycin, and spectinomycin (100% each), while 60.5% and 43.4% of the examined isolates had high minimum inhibitory concentration (MIC) values to enrofloxacin and doxycycline, respectively. Three and two mycoplasma isolates with high enrofloxacin MICs were confirmed to be M. bovis and M. bovirhinis, respectively, by PCR assays. All molecularly confirmed mycoplasma isolates (n = 5) were positive for the gyrA gene (100%); meanwhile, three isolates (60%) were positive for the parC gene. In conclusion, our findings revealed alarming resistance to enrofloxacin and doxycycline antibiotics; thus, antimicrobial usage must be restricted and molecular techniques can help in the rapid detection of the resistant strains.
Keywords: Mycoplasm bovis; cattle; fluoroquinolone resistance; gyrA; parC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Short communication: In vitro antimicrobial susceptibility of Mycoplasma bovis isolates identified in milk from dairy cattle in Belgium, Germany, and Italy.J Dairy Sci. 2016 Aug;99(8):6578-6584. doi: 10.3168/jds.2015-10572. Epub 2016 May 18. J Dairy Sci. 2016. PMID: 27209138
-
Antimicrobial susceptibility and molecular characteristics of Mycoplasma bovis isolated from cases of bovine respiratory disease in Australian feedlot cattle.Vet Microbiol. 2023 Aug;283:109779. doi: 10.1016/j.vetmic.2023.109779. Epub 2023 May 23. Vet Microbiol. 2023. PMID: 37257307
-
Antimicrobial susceptibility of Mycoplasma bovis isolates from veal calves and dairy cattle in the Netherlands.Vet Microbiol. 2016 Jun 30;189:1-7. doi: 10.1016/j.vetmic.2016.04.012. Epub 2016 Apr 11. Vet Microbiol. 2016. PMID: 27259820
-
Mycoplasma bovis: Mechanisms of Resistance and Trends in Antimicrobial Susceptibility.Front Microbiol. 2016 Apr 27;7:595. doi: 10.3389/fmicb.2016.00595. eCollection 2016. Front Microbiol. 2016. PMID: 27199926 Free PMC article. Review.
-
Integrating the Human and Animal Sides of Mycoplasmas Resistance to Antimicrobials.Antibiotics (Basel). 2021 Oct 7;10(10):1216. doi: 10.3390/antibiotics10101216. Antibiotics (Basel). 2021. PMID: 34680797 Free PMC article. Review.
Cited by
-
Future impact of thymoquinone-loaded nanoemulsion in rabbits: prospects for enhancing growth, immunity, antioxidant potential and resistance against Pasteurella multocida.Front Vet Sci. 2024 Jan 16;10:1340964. doi: 10.3389/fvets.2023.1340964. eCollection 2023. Front Vet Sci. 2024. PMID: 38292130 Free PMC article.
-
Editorial for the Special Issue "Microbial Diversity and Microbial Resistance".Biology (Basel). 2025 Apr 13;14(4):415. doi: 10.3390/biology14040415. Biology (Basel). 2025. PMID: 40282279 Free PMC article.
-
A Systematic Review of the Recent Techniques Commonly Used in the Diagnosis of Mycoplasma bovis in Dairy Cattle.Pathogens. 2023 Sep 19;12(9):1178. doi: 10.3390/pathogens12091178. Pathogens. 2023. PMID: 37764986 Free PMC article. Review.
-
Combined modulatory effects of dietary arginine and olive leaf phenolic extract on growth performance and immune functions of broiler chickens, and meat antioxidant potential during frozen storage.BMC Vet Res. 2025 Mar 31;21(1):226. doi: 10.1186/s12917-025-04663-6. BMC Vet Res. 2025. PMID: 40165253 Free PMC article.
-
A Comprehensive Review of the Common Bacterial Infections in Dairy Calves and Advanced Strategies for Health Management.Vet Med (Auckl). 2024 Jan 24;15:1-14. doi: 10.2147/VMRR.S452925. eCollection 2024. Vet Med (Auckl). 2024. PMID: 38288284 Free PMC article. Review.
References
-
- Isa F.M. Mycoplasma bovis: A Neglected Pathogen in Nigeria-A Mini Review. Dairy Vet. Sci. J. 2017;4:555633.
-
- Hale H.H., Helmboldt C.F., Plastridge W.N., Stula E.F. Bovine mastitis caused by a Mycoplasma spp. Cornell Vet. 1962;52:582–591. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

