Glycosaminoglycan linkage region of urinary bikunin as a potentially useful biomarker for β3GalT6-deficient spondylodysplastic Ehlers-Danlos syndrome
- PMID: 36101818
- PMCID: PMC9458601
- DOI: 10.1002/jmd2.12311
Glycosaminoglycan linkage region of urinary bikunin as a potentially useful biomarker for β3GalT6-deficient spondylodysplastic Ehlers-Danlos syndrome
Abstract
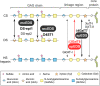
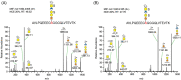
The spondylodysplastic type of Ehlers-Danlos syndrome (spEDS) is caused by genetic defects in the B4GALT7 or B3GALT6 genes both deranging the biosynthesis of the glycosaminoglycan linkage region of chondroitin/dermatan sulfate and heparan sulfate proteoglycans. In this study, we have analyzed the linkage regions of urinary chondroitin sulfate proteoglycans of three siblings, diagnosed with spEDS and carrying biallelic pathogenic variants of the B3GALT6 gene. Proteoglycans were digested with trypsin, glycopeptides enriched on anion-exchange columns, depolymerized with chondroitinase ABC, and analyzed by nLC-MS/MS. In urine of the unaffected mother, the dominating glycopeptide of bikunin/protein AMBP appeared as only one dominating (99.9%) peak with the canonical tetrasaccharide linkage region modification. In contrast, the samples of the three affected siblings contained two different glycopeptide peaks, corresponding to the canonical tetrasaccharide and to the non-canonical trisaccharide linkage region modifications in individual ratios of 61/38, 73/27, and 59/41. We propose that the relative distribution of glycosaminoglycan linkage regions of urinary bikunin glycopeptides may serve as a phenotypic biomarker in a diagnostic test but also as a biomarker to follow the effect of future therapies in affected individuals.
Keywords: Ehlers–Danlos syndrome; bikunin; glycopeptides; glycoproteomics; glycosaminoglycan linkage region; linkeropathies; liquid chromatography; tandem mass spectrometry; β3GalT6.
© 2022 The Authors. JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Mahnaz Nikpour, Fredrik Noborn, Jonas Nilsson, Tim Van Damme, Olivier Kaye, Delfien Syx, Fransiska Malfait, and Göran Larson all declare that they have no conflict of interest.
Figures
References
-
- Bai X, Zhou D, Brown JR, Crawford BE, Hennet T, Esko JD. Biosynthesis of the linkage region of glycosaminoglycans: cloning and activity of galactosyltransferase II, the sixth member of the beta 1,3‐galactosyltransferase family (beta 3GalT6). J Biol Chem. 2001;276:48189‐48195. - PubMed
-
- Delbaere S, Van Damme T, Syx D, et al. Hypomorphic zebrafish models mimic the musculoskeletal phenotype of beta 4GalT7‐deficient Ehlers‐Danlos syndrome. Matrix Biol. 2020b;89:59‐75. - PubMed
-
- Esko JD, Kimata K, Lindahl U. Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, et al., eds. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; 2009. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

