Impaired Dynamic Sarcoplasmic Reticulum Ca Buffering in Autosomal Dominant CPVT2
- PMID: 36102198
- PMCID: PMC9529867
- DOI: 10.1161/CIRCRESAHA.121.320661
Impaired Dynamic Sarcoplasmic Reticulum Ca Buffering in Autosomal Dominant CPVT2
Abstract
Background: Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a potentially lethal cardiac arrhythmia syndrome triggered by catecholamines released during exercise, stress, or sudden emotion. Variants in the calsequestrin-2 gene (CASQ2), encoding the major calcium (Ca) binding protein in the sarcoplasmic reticulum (SR), are the second most common cause of CPVT. Recently, several CASQ2 gene variants, such as CASQ2-K180R, have been linked to an autosomal dominant form of Casq2-linked CPVT (CPVT2), but the underlying mechanism is not known.
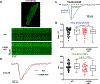
Methods: A K180R mouse model was generated using CRIPSR/Cas9. Heterozygous and homozygous K180R mice were studied using telemetry ECG recordings in vivo. Ventricular cardiomyocytes were isolated and studied using fluorescent Ca indicators and patch clamp. Expression levels and localization of SR Ca-handling proteins were evaluated using Western blotting and immunostaining. Intra-SR Ca kinetics were quantified using low-affinity Ca indicators.
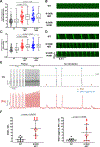
Results: K180R mice exhibit an autosomal dominant CPVT phenotype following exercise or catecholamine stress. Upon catecholamine stress, K180R ventricular cardiomyocytes exhibit increased spontaneous SR Ca release events, triggering delayed afterdepolarizations and spontaneous beats. K180R had no effect on levels of Casq2, Casq2 polymers, or other SR Ca-handling proteins. Intra-SR Ca measurements revealed that K180R impaired dynamic intra-SR Ca buffering, resulting in a more rapid rise of free Ca in the SR during diastole. Steady-state SR Ca buffering and total SR Ca content were not changed. Consistent with the reduced dynamic intra-SR buffering, K180R causes reduced SR Ca release refractoriness.
Conclusions: CASQ2-K180R causes CPVT2 via a heretofore unknown mechanism that differs from CASQ2 variants associated with autosomal recessive CPVT2. Unlike autosomal recessive CASQ2 variants, K180R impairs the dynamic buffering of Ca within the SR without affecting total SR Ca content or Casq2 protein levels. Our data provide insight into the molecular mechanism underlying autosomal dominant CPVT2.
Keywords: calcium; calsequestrin; catecholamine; sarcoplasmic reticulum; tachycardia.
Conflict of interest statement
Disclosures
The authors have declared that no conflict of interest exists.
Figures

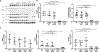

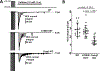


References
-
- Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 1995;91:1512–1519. - PubMed
-
- Roston TM, Yuchi Z, Kannankeril PJ, Hathaway J, Vinocur JM, Etheridge SP, Potts JE, Maginot KR, Salerno JC, Cohen MI, et al. The clinical and genetic spectrum of catecholaminergic polymorphic ventricular tachycardia: findings from an international multicentre registry. Europace 2018;20:541–547. doi: 10.1093/europace/euw389 - DOI - PMC - PubMed
-
- Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet 2001;69:1378–1384. - PMC - PubMed
-
- Lahat H, Eldar M, Levy-Nissenbaum E, Bahan T, Friedman E, Khoury A, Lorber A, Kastner DL, Goldman B, Pras E. Autosomal recessive catecholamine- or exercise-induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome 1p13–21. Circulation 2001;103:2822–2827. doi: 10.1161/01.cir.103.23.2822 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

