From Local Covalent Bonding to Extended Electric Field Interactions in Proton Hydration
- PMID: 36102247
- PMCID: PMC9827956
- DOI: 10.1002/anie.202211066
From Local Covalent Bonding to Extended Electric Field Interactions in Proton Hydration
Abstract
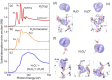
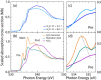
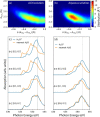
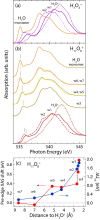
Seemingly simple yet surprisingly difficult to probe, excess protons in water constitute complex quantum objects with strong interactions with the extended and dynamically changing hydrogen-bonding network of the liquid. Proton hydration plays pivotal roles in energy transport in hydrogen fuel cells and signal transduction in transmembrane proteins. While geometries and stoichiometry have been widely addressed in both experiment and theory, the electronic structure of these specific hydrated proton complexes has remained elusive. Here we show, layer by layer, how utilizing novel flatjet technology for accurate x-ray spectroscopic measurements and combining infrared spectral analysis and calculations, we find orbital-specific markers that distinguish two main electronic-structure effects: Local orbital interactions determine covalent bonding between the proton and neigbouring water molecules, while orbital-energy shifts measure the strength of the extended electric field of the proton.
Keywords: Eigen Cation; Electronic Structure; Hydrated Proton; Soft X-Ray Absorption Spectroscopy; Zundel Cation.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Marx D., Tuckerman M. E., Hutter J., Parrinello M., Nature 1999, 397, 601–604.
-
- Eigen M., Angew. Chem. Int. Ed. Engl. 1964, 3, 1–19;
- Angew. Chem. 1963, 75, 489–508.
-
- None
-
- Hickner M. A., Ghassemi H., Kim Y. S., Einsla B. R., McGrath J. E., Chem. Rev. 2004, 104, 4587–4611; - PubMed
-
- Kreuer K.-D., Paddison S. J., Spohr E., Schuster M., Chem. Rev. 2004, 104, 4637–4678. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

