Assessment of Cardiac, Vascular, and Pulmonary Pathobiology In Vivo During Acute COVID-19
- PMID: 36102258
- PMCID: PMC9683664
- DOI: 10.1161/JAHA.122.026399
Assessment of Cardiac, Vascular, and Pulmonary Pathobiology In Vivo During Acute COVID-19
Abstract
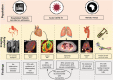
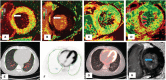
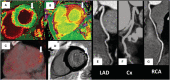
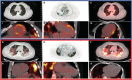
Background Acute COVID-19-related myocardial, pulmonary, and vascular pathology and how these relate to each other remain unclear. To our knowledge, no studies have used complementary imaging techniques, including molecular imaging, to elucidate this. We used multimodality imaging and biochemical sampling in vivo to identify the pathobiology of acute COVID-19. Specifically, we investigated the presence of myocardial inflammation and its association with coronary artery disease, systemic vasculitis, and pneumonitis. Methods and Results Consecutive patients presenting with acute COVID-19 were prospectively recruited during hospital admission in this cross-sectional study. Imaging involved computed tomography coronary angiography (identified coronary disease), cardiac 2-deoxy-2-[fluorine-18]fluoro-D-glucose positron emission tomography/computed tomography (identified vascular, cardiac, and pulmonary inflammatory cell infiltration), and cardiac magnetic resonance (identified myocardial disease) alongside biomarker sampling. Of 33 patients (median age 51 years, 94% men), 24 (73%) had respiratory symptoms, with the remainder having nonspecific viral symptoms. A total of 9 patients (35%, n=9/25) had cardiac magnetic resonance-defined myocarditis. Of these patients, 53% (n=5/8) had myocardial inflammatory cell infiltration. A total of 2 patients (5%) had elevated troponin levels. Cardiac troponin concentrations were not significantly higher in patients with and without myocarditis (8.4 ng/L [interquartile range, IQR: 4.0-55.3] versus 3.5 ng/L [IQR: 2.5-5.5]; P=0.07) or myocardial cell infiltration (4.4 ng/L [IQR: 3.4-8.3] versus 3.5 ng/L [IQR: 2.8-7.2]; P=0.89). No patients had obstructive coronary artery disease or vasculitis. Pulmonary inflammation and consolidation (percentage of total lung volume) was 17% (IQR: 5%-31%) and 11% (IQR: 7%-18%), respectively. Neither were associated with the presence of myocarditis. Conclusions Myocarditis was present in a third patients with acute COVID-19, and the majority had inflammatory cell infiltration. Pneumonitis was ubiquitous, but this inflammation was not associated with myocarditis. The mechanism of cardiac pathology is nonischemic and not attributable to a vasculitic process. Registration URL: https://www.isrctn.com; Unique identifier: ISRCTN12154994.
Keywords: CMR; COVID‐19; FDG‐PET; myocarditis; pneumonitis.
Figures
References
-
- Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa‐Nicotera M, Zeiher AM, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557 - DOI - PMC - PubMed
-
- Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, et al. Patterns of myocardial injury in recovered troponin‐positive COVID‐19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/eurheartj/ehab075 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

