Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial
- PMID: 36102337
- PMCID: PMC10011015
- DOI: 10.1002/alz.12767
Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial
Abstract
Introduction: Dietary supplements are touted for cognitive protection, but supporting evidence is mixed. COSMOS-Mind tested whether daily administration of cocoa extract (containing 500 mg/day flavanols) versus placebo and a commercial multivitamin-mineral (MVM) versus placebo improved cognition in older women and men.
Methods: COSMOS-Mind, a large randomized two-by-two factorial 3-year trial, assessed cognition by telephone at baseline and annually. The primary outcome was a global cognition composite formed from mean standardized (z) scores (relative to baseline) from individual tests, including the Telephone Interview of Cognitive Status, Word List and Story Recall, Oral Trail-Making, Verbal Fluency, Number Span, and Digit Ordering. Using intention-to-treat, the primary endpoint was change in this composite with 3 years of cocoa extract use. The pre-specified secondary endpoint was change in the composite with 3 years of MVM supplementation. Treatment effects were also examined for executive function and memory composite scores, and in pre-specified subgroups at higher risk for cognitive decline.
Results: A total of 2262 participants were enrolled (mean age = 73y; 60% women; 89% non-Hispanic White), and 92% completed the baseline and at least one annual assessment. Cocoa extract had no effect on global cognition (mean z-score = 0.03, 95% CI: -0.02 to 0.08; P = .28). Daily MVM supplementation, relative to placebo, resulted in a statistically significant benefit on global cognition (mean z = 0.07, 95% CI 0.02 to 0.12; P = .007), and this effect was most pronounced in participants with a history of cardiovascular disease (no history: 0.06, 95% CI 0.01 to 0.11; history: 0.14, 95% CI -0.02 to 0.31; interaction, nominal P = .01). Multivitamin-mineral benefits were also observed for memory and executive function. The cocoa extract by MVM group interaction was not significant for any of the cognitive composites.
Discussion: Cocoa extract did not benefit cognition. However, COSMOS-Mind provides the first evidence from a large, long-term, pragmatic trial to support the potential efficacy of a MVM to improve cognition in older adults. Additional work is needed to confirm these findings in a more diverse cohort and to identify mechanisms to account for MVM effects.
Highlights: COSMOS-Mind was a large simple pragmatic randomized clinical trial in older adults conducted by mail and telephone. The trial used a two-by-two factorial design to assess treatment effects of two different interventions within a single large study. We found no cognitive benefit of daily cocoa extract administration (containing 500 mg flavanols) for 3 years. Daily multivitamin-mineral (MVM) supplementation for 3 years improved global cognition, episodic memory, and executive function in older adults. The MVM benefit appeared to be greater for adults with cardiovascular disease.
Keywords: aging; clinical trial; cocoa extract; cognition; cognitive function; flavanols; multivitamin; older adults.
© 2022 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Competing Interests
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health and the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Neither the National Institutes on Health, Mars, nor Pfizer contributed to any aspect of the trial including design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The authors have no competing interests to report.
Figures

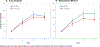
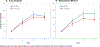

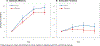
References
-
- Prince M, Wimo A, Guerchet M, Ali GC, Wu Y, M. P World Alzheimer Report 2015. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International;2015.
-
- Cavazzoni P FDA’s Decision to Approve New Treatment for Alzheimer’s Disease. In: Administration USFD, ed2021.
-
- NIA. Living Long & Well in the 21st Century: Strategic Directions for Research on Aging. 2014.
-
- Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119(10):1433–1441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

