Periostin- and podoplanin-positive cancer-associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma
- PMID: 36102377
- PMCID: PMC9828775
- DOI: 10.1002/path.6011
Periostin- and podoplanin-positive cancer-associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma
Abstract
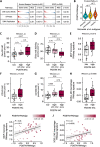
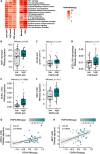
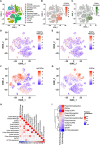
Cancer-associated fibroblasts (CAFs) are orchestrators of the pancreatic ductal adenocarcinoma (PDAC) microenvironment. Previously we described four CAF subtypes with specific molecular and functional features. Here, we have refined our CAF subtype signatures using RNAseq and immunostaining with the goal of defining bioinformatically the phenotypic stromal and tumor epithelial states associated with CAF diversity. We used primary CAF cultures grown from patient PDAC tumors, human data sets (in-house and public, including single-cell analyses), genetically engineered mouse PDAC tissues, and patient-derived xenografts (PDX) grown in mice. We found that CAF subtype RNAseq signatures correlated with immunostaining. Tumors rich in periostin-positive CAFs were significantly associated with shorter overall survival of patients. Periostin-positive CAFs were characterized by high proliferation and protein synthesis rates and low α-smooth muscle actin expression and were found in peri-/pre-tumoral areas. They were associated with highly cellular tumors and with macrophage infiltrates. Podoplanin-positive CAFs were associated with immune-related signatures and recruitment of dendritic cells. Importantly, we showed that the combination of periostin-positive CAFs and podoplanin-positive CAFs was associated with specific tumor microenvironment features in terms of stromal abundance and immune cell infiltrates. Podoplanin-positive CAFs identified an inflammatory CAF (iCAF)-like subset, whereas periostin-positive CAFs were not correlated with the published myofibroblastic CAF (myCAF)/iCAF classification. Taken together, these results suggest that a periostin-positive CAF is an early, activated CAF, associated with aggressive tumors, whereas a podoplanin-positive CAF is associated with an immune-related phenotype. These two subpopulations cooperate to define specific tumor microenvironment and patient prognosis and are of putative interest for future therapeutic stratification of patients. © 2022 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: RNAseq; dendritic cell; heterogeneity; immune cells; immunohistochemistry; macrophage; prognosis; stroma; transcriptomic classification.
© 2022 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Figures



References
-
- Neesse A, Bauer CA, Öhlund D, et al. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut 2019; 68: 159–171. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

