Atrial Flutter in PRKAG2 Syndrome: Clinical and Electrophysiological Characteristics
- PMID: 36102422
- PMCID: PMC9750222
- DOI: 10.36660/abc.20210792
Atrial Flutter in PRKAG2 Syndrome: Clinical and Electrophysiological Characteristics
Abstract
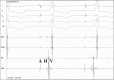
Background: PRKAG2 syndrome is a rare autosomal dominant disease, a phenocopy of hypertrophic cardiomyopathy characterized by intracellular glycogen accumulation. Clinical manifestations include ventricular preexcitation, cardiac conduction disorder, ventricular hypertrophy, and atrial arrhythmias.
Objective: To compare the clinical and electrophysiological characteristics observed in patients with atrial flutter, with and without PRKAG2 syndrome.
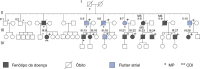
Methods: An observational study comparing patients with atrial flutter: group A consisted of five patients with PRKAG2 syndrome from a family, and group B consisted of 25 patients without phenotype of PRKAG2 syndrome. The level of significance was 5%.
Results: All patients in group A had ventricular preexcitation and right branch block, and four had pacemakers (80%). Patients in group A were younger (39±5.4 vs 58.6±17.6 years, p=0.021), had greater interventricular septum (median=18 vs 10 mm; p<0.001) and posterior wall thickness (median=14 vs 10 mm; p=0.001). In group A, four patients were submitted to an electrophysiological study, showing a fasciculoventricular pathway, and atrial flutter ablation was performed in tree. All patients in group B were submitted to ablation of atrial flutter, with no evidence of accessory pathway. Group B had a higher prevalence of hypertension, diabetes mellitus, coronary artery disease and sleep apnea, with no statistically significant difference.
Conclusion: Patients with PRKAG2 syndrome presented atrial flutter at an earlier age and had fewer comorbidities when compared to patients with atrial flutter without mutation phenotype. The occurrence of atrial flutter in young individuals, especially in the presence of ventricular preexcitation and familial ventricular hypertrophy, should raise the suspicion of PRKAG2 syndrome.
Fundamento: A síndrome do PRKAG2 é uma rara doença genética autossômico dominante, fenocópia da miocardiopatia hipertrófica, caracterizada pelo acúmulo intracelular de glicogênio. Manifestações clínicas incluem pré-excitação ventricular, hipertrofia ventricular, distúrbio de condução cardíaca e arritmias atriais.
Objetivo: Comparar características clínicas e eletrofisiológicas observadas em pacientes com flutter atrial, com e sem síndrome do PRKAG2.
Métodos: Estudo observacional, comparativo de pacientes com flutter atrial: grupo A, cinco pacientes de família com síndrome do PRKAG2; e grupo B, 25 pacientes sem fenótipo da síndrome. O nível de significância foi de 5%.
Resultados: Todos os pacientes do grupo A apresentaram pré-excitação ventricular e bloqueio de ramo direito; quatro tinham marca-passo (80%). Pacientes do grupo A tinham menor idade (39±5,4 vs. 58,6±17,6 anos, p=0,021), e maior espessura de septo interventricular (mediana=18 vs. 10 mm; p<0,001) e parede posterior (mediana=14 vs. 10 mm; p=0,001). Quatro do grupo A foram submetidos a estudo eletrofisiológico, sendo observada via acessória fascículo-ventricular; em três foi realizada ablação do flutter atrial. Todos os do grupo B foram submetidos à ablação do flutter atrial, sem evidência de via acessória. Observado maior prevalência no grupo B de hipertensão arterial, diabetes mellitus, doença coronariana e apneia do sono, sem diferença estatisticamente significante.
Conclusão: Portadores da síndrome do PRKAG2 apresentaram flutter atrial em idade mais precoce, e menos comorbidades, quando comparados a pacientes com flutter atrial sem fenótipo da mutação. Importante suspeitar de miocardiopatia geneticamente determinada, como síndrome do PRKAG2, em jovens com flutter atrial, especialmente na presença de pré-excitação ventricular e hipertrofia ventricular familiar.
Conflict of interest statement
Potencial conflito de interesse
Não há conflito com o presente artigo.
Figures




Comment in
-
PRKAG2 Cardiomyopathy.Arq Bras Cardiol. 2022 Nov;119(5):689-690. doi: 10.36660/abc.20220694. Arq Bras Cardiol. 2022. PMID: 36453759 Free PMC article. English, Portuguese. No abstract available.
References
-
- Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, et al. Identification of a gene responsible for familial Wolff-Parkinson-white syndrome. N Engl J Med. 2001;344:1823–1831. - PubMed
LinkOut - more resources
Full Text Sources

