Immune Imprinting Drives Human Norovirus Potential for Global Spread
- PMID: 36102514
- PMCID: PMC9600701
- DOI: 10.1128/mbio.01861-22
Immune Imprinting Drives Human Norovirus Potential for Global Spread
Abstract
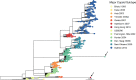
Understanding the complex interactions between virus and host that drive new strain evolution is key to predicting the emergence potential of variants and informing vaccine development. Under our hypothesis, future dominant human norovirus GII.4 variants with critical antigenic properties that allow them to spread are currently circulating undetected, having diverged years earlier. Through large-scale sequencing of GII.4 surveillance samples, we identified two variants with extensive divergence within domains that mediate neutralizing antibody binding. Subsequent serological characterization of these strains using temporally resolved adult and child sera suggests that neither candidate could spread globally in adults with multiple GII.4 exposures, yet young children with minimal GII.4 exposure appear susceptible. Antigenic cartography of surveillance and outbreak sera indicates that continued population exposure to GII.4 Sydney 2012 and antigenically related variants over a 6-year period resulted in a broadening of immunity to heterogeneous GII.4 variants, including those identified here. We show that the strongest antibody responses in adults exposed to GII.4 Sydney 2012 are directed to previously circulating GII.4 viruses. Our data suggest that the broadening of antibody responses compromises establishment of strong GII.4 Sydney 2012 immunity, thereby allowing the continued persistence of GII.4 Sydney 2012 and modulating the cycle of norovirus GII.4 variant replacement. Our results indicate a cycle of norovirus GII.4 variant replacement dependent upon population immunity. Young children are susceptible to divergent variants; therefore, emergence of these strains worldwide is driven proximally by changes in adult serological immunity and distally by viral evolution that confers fitness in the context of immunity. IMPORTANCE In our model, preepidemic human norovirus variants harbor genetic diversification that translates into novel antigenic features without compromising viral fitness. Through surveillance, we identified two viruses fitting this profile, forming long branches on a phylogenetic tree. Neither evades current adult immunity, yet young children are likely susceptible. By comparing serological responses, we demonstrate that population immunity varies by age/exposure, impacting predicted susceptibility to variants. Repeat exposure to antigenically similar variants broadens antibody responses, providing immunological coverage of diverse variants but compromising response to the infecting variant, allowing continued circulation. These data indicate norovirus GII.4 variant replacement is driven distally by virus evolution and proximally by immunity in adults.
Keywords: antigenic cartography; antigenic seniority; epidemic; histo-blood group antigens; immune imprinting; neutralizing antibodies; norovirus; sequencing; surveillance; variant persistence; variants of concern.
Conflict of interest statement
The authors declare a conflict of interest. L.C.L. and R.S.B. hold patents on norovirus vaccine design and ongoing collaborations with VaxArt, Takeda Vaccines and HilleVax that are unrelated and do not pose conflicts of interest with this report. R.S.B. is a member of the advisory committee for VaxArt and Adagio Therapeutics. S.B.D. received an investigator-initiated research award from Takeda Vaccines unrelated to this report. P.B.J., M.L.M., M.R.Z., H.C., S.R.M., F.A.T.B., R.S., H.T., R.W., R.G., J.B., D.J.A., D.K., K.O., F.B., J.V., V.C., C.C.C., and S.B. have no conflicts of interest.
Figures




References
-
- Sherwood J, Mendelman PM, Lloyd E, Liu M, Boslego J, Borkowski A, Jackson A, Faix D, US Navy study team . 2020. Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults. Vaccine 38:6442–6449. doi:10.1016/j.vaccine.2020.07.069. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
