E2/E3-independent ubiquitin-like protein conjugation by Urm1 is directly coupled to cysteine persulfidation
- PMID: 36102610
- PMCID: PMC9574740
- DOI: 10.15252/embj.2022111318
E2/E3-independent ubiquitin-like protein conjugation by Urm1 is directly coupled to cysteine persulfidation
Abstract
Post-translational modifications by ubiquitin-like proteins (UBLs) are essential for nearly all cellular processes. Ubiquitin-related modifier 1 (Urm1) is a unique UBL, which plays a key role in tRNA anticodon thiolation as a sulfur carrier protein (SCP) and is linked to the noncanonical E1 enzyme Uba4 (ubiquitin-like protein activator 4). While Urm1 has also been observed to conjugate to target proteins like other UBLs, the molecular mechanism of its attachment remains unknown. Here, we reconstitute the covalent attachment of thiocarboxylated Urm1 to various cellular target proteins in vitro, revealing that, unlike other known UBLs, this process is E2/E3-independent and requires oxidative stress. Furthermore, we present the crystal structures of the peroxiredoxin Ahp1 before and after the covalent attachment of Urm1. Surprisingly, we show that urmylation is accompanied by the transfer of sulfur to cysteine residues in the target proteins, also known as cysteine persulfidation. Our results illustrate the role of the Uba4-Urm1 system as a key evolutionary link between prokaryotic SCPs and the UBL modifications observed in modern eukaryotes.
Keywords: Urm1; oxidative stress; persulfidation; sulfur transfer; ubiquitin-like.
© 2022 The Authors. Published under the terms of the CC BY NC ND 4.0 license.
Figures

- A
Scheme of different methodologies to generate Urm1‐SH in vitro. Sulfur from cysteine is passed from Nfs1 to Uba4 directly or via Tum1. Uba4 transfers sulfur to Urm1 by catalyzing sequential adenylation and thiocarboxylation reactions. The intein‐based inducible cleavage system can produce carboxylated Urm1 (OH) or thiocarboxylated Urm1 (SH) by using DTT or ammonium sulfide (NH4)2S, respectively. Intein‐produced SH is in orange and Uba4‐produced SH* is in blue.
- B
ESI‐MS analyses of Urm1‐OH and Urm1‐SH produced via Uba4 (blue and asterisk) or the intein system (orange). The expected and assessed masses of the samples are listed. Inset: APM gel analyses of the same Urm1 samples. SDS–PAGE gel below for the same proteins using a sample buffer containing DTT. APM: [(N‐acryloylamino)phenyl]mercuric chloride. SDS–PAGE gels were stained with Coomassie.
- C
In vivo conjugation analyses in the presence (left) or absence of NEM (N‐Ethylmaleimide) (right) with protein extracts obtained from the indicated yeast strains expressing HA‐URM1. Unconjugated Urm1 and Urm1‐conjugates were detected by anti‐HA (top panels) Western blots. Anti‐Ahp1 blots (middle) detect unconjugated Ahp1. Anti‐Cdc19 (bottom panels) served as a loading control.
- D
Analysis of covalent adduct formation between ScAhp1 or ScAhp1C31S or ScAhp1C31S C62S mutant and carboxylated (OH) or thiocarboxylated ScUrm1 (SH) or Uba4 produced thiocarboxylated Urm1 (SH*) in the presence of TBH (tert‐Butyl hydroperoxide). Confirmation of the isopeptide bond was carried out by the addition of DTT, TCEP, or HA, respectively. Unconjugated Ahp1 and Urm1 as well as their formed conjugate (Ahp1‐Urm1) are indicated on the right. DTT: 1,4‐Dithiothreitol; TCEP: Tris(2‐carboxyethyl) phosphine; HA: Hydroxylamine.
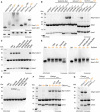
- A
Analysis of thiocarboxylated Urm1 generated using the intein system by APM gel electrophoresis and retardation. HsUrm1, CtUrm1, the cysteine mutant CtUrm1C55S, and CtUrm1C55S‐G111C are examined. Standard SDS page gel below for the same proteins with sample buffer containing DTT. Coomassie was used for staining.
- B
Analysis of covalent adduct formation between ScAhp1 or ScAhp1C31S or ScAhp1C31R or ScAhp1C62S or ScAhp1C31S/C62S mutant and thiocarboxylated ScUrm1 (SH) or carboxylated ScUrm1 (OH) in the presence of TBH (tert‐Butyl hydroperoxide). Analysis of covalent adduct formation between CtAhp1 or CtAhp1C30S or CtAhp1C60S or CtAhp1C30S/60S mutant and thiocarboxylated CtUrm1C55S (SH) in the presence of TBH.
- C
Analysis of isopeptide‐bond formation between ScAhp1C31S mutant and thiocarboxylated ScUrm1 in the absence or presence of different reactive species (RS) inducing chemicals such as TBH, HP, Diamide, Peroxynitrite, Methylglyoxal, and DTB‐Disulfide. NEM was added either before adding TBH or after (NEM*). HP: Hydrogen peroxide; DTB: Disulfide‐Di‐tert‐butyl disulfide; NEM: N‐Ethylmaleimide.
- D
Analysis of ScAhp1C31S stability treated with TBH, HP, Diamide, Peroxynitrite, Methylglyoxal, DTB‐Disulfide, and NEM.
- E
Analysis of the stability of the thiocarboxylated ScUrm1 subjected to various reactive species producing chemicals such as TBH, HP, Diamide, Peroxynitrite, Methylglyoxal, and DTB‐Disulfide. ScUrm1‐COSH subjected to NEM was also analyzed.
- F
Analysis of covalent adduct formation between CtAhp1 or CtAhp1C30S mutant and thiocarboxylated CtUrm1C55S or carboxylated (OH) in the presence of TBH. Confirmation of an isopeptide bond was conducted by using DTT/TCEP/HA.
- G
Analysis of covalent adduct formation between CtAhp1C30S and CtUrm1‐SH or CtUrm1C55S‐SH or CtUrm1G111C mutant in the presence of TBH.

Size‐exclusion chromatography profiles of CtAhp1C30S (yellow), CtUrm1C55S (cyan) and the CtAhp1C30S‐CtUrm1C55S complex (pink). SDS–PAGE analysis of the CtAhp1C30S‐CtUrm1C55S complex and individual proteins CtAhp1C30S and CtUrm1C55S‐SH. Equivalent fractions of each run were collected and analyzed separately. The expected molecular mass of the individual samples are as follows—CtUrm1C55S‐SH 12.5 kDa; CtAhp1C30S 18 kDa; CtAhp1C30S‐CtUrm1C55S complex 30.5 kDa.
Crystal structures of dimeric CtAhp1 and CtAhp1C30S in cartoon representation. Insets: Close‐up of the active sites highlighting redox‐active cysteines (Cys60 and Cys/Ser30 in red).
Front (up) and top (down) view of the CtAhp1C30S‐CtUrm1C55S complex structure in cartoon representation. All three entities in the asymmetric unit are superimposed on Ahp1 and the various Urm1 molecules are labeled. The position of Lys63 (violet) is highlighted and labeled. Insets: Close‐up of the active sites highlighting the position of the redox‐active cysteines (Cys60 and Ser30 in red).
Comparison of the three observed states of Urm1 conjugation in relation to the linkage site of Ahp1. All 6 Ahp‐Urm1 entities in the asymmetric unit are superimposed on Ahp1. All relevant residues are labeled and highlighted in the ball and stick representation. The C‐terminal residues of the attached Urm1 are shown in cartoon representation and the remaining parts as a transparent surface. Cysteines are highlighted (Cys60 and Ser30 in red).

- A
Comparison of the individual Ahp1‐Urm1 units in the asymmetric unit. Close‐up of the formed peptide bond with all nearby residues labeled and highlighted in a balls‐and‐sticks representation (Cys60 in red). The last strand of the attached Urm1 protein is shown in cartoon representation and the refined 2Fo‐Fc density (blue) and the Fo‐Fc omit map lacking the highlighted residues (green) is shown around Ahp1Lys63 and Urm1Gly111 at 1σ and 3σ, respectively.
- B
Top: MS/MS spectrum of the K63‐ε‐GGH containing peptide from CtAhp1C30S and K32‐ε‐GGH containing peptide from ScAhp1C31S. A representative annotated fragmentation spectrum is shown with the b‐ and y‐ions marked in red and blue, respectively. The precursor ions are labeled in green. The peptide sequence is shown at the top with the collision‐induced fragmentation pattern. m/z: mass to charge ratio. Bottom: Schematic summary of the detected conjugated peptides by mass spectrometry for CtAhp1C30S, CtAhp1C30S K63A, and ScAhp1C31S. The thickness of the line represents the number of replicates where the respective site was detected, and the height represents the associated maximum Mascot ion score (n ≥ 3). The urmylation was identified as HisGlyGly tag on lysines, serines, and threonines while persulfidation was searched as a mass shift: +32 Da (persulfidation) or +89 Da (persulfidation + carbamidomethylation) on cysteines.
- C
Analysis of conjugation between CtAhp1C30S variants in combination with thiocarboxylated CtUrm1C55S in the presence of TBH. Unconjugated Ahp1 and Urm1 as well as the formed conjugates (Ahp1‐Urm1) are indicated on the right.
- D
Analysis of conjugation between ScAhp1C31S variants in combination with thiocarboxylated ScUrm1 in the presence of TBH. Unconjugated Ahp1 and Urm1 as well as the formed conjugates (Ahp1‐Urm1) are indicated on the right. ScAhp14KR: ScAhp1C31S K47/48R K124R K156R; ScAhp15KR: ScAhp1C31S K32R K47/48R K124R K156R.
- E
TBH cytotoxicity assay in vivo of the ScAhp1 lysine mutants in yeast. Growth of yap1Δahp1Δ cells carrying empty vector (ev), wild‐type peroxiredoxin gene (AHP1), or indicated cysteine or lysine substitutions was monitored without or with 1 mM TBH. ScAhp14KR: ScAhp1C31S K47/48R K124R K156R; ScAhp15KR: ScAhp1C31S K32R K47/48R K124R K156R.
- F
In vivo conjugation analyses of ScAhp1 lysine mutants with protein extracts obtained from the indicated yeast strains expressing HA‐URM1. Unconjugated Urm1 and Urm1‐Ahp1 conjugates were detected by anti‐HA (top panels) Western blots. Anti‐Ahp1 blots (middle) detect unmodified Ahp1. Anti‐Cdc19 (bottom panels) served as a loading control. ScAhp14KR: ScAhp1C31S K47/48R K124R K156R; ScAhp15KR: ScAhp1C31S K32R K47/48R K124R K156R.
- G
Comparison of the crystal structures of the oxidized dimeric forms of Ahp1 from CtAhp1C30S (left) and ScAhp1 (right; PDB ID 4DSR; Lian et al, 2012). All lysine residues (green) are shown in the ball and sticks representation. Conjugated lysine residues detected by mass spectrometry (in B) are labeled and highlighted in black. Other lysine residues are labeled in gray and peroxidatic cysteines (Cys60 in CtAhp1 and Cys62 in ScAhp1) and their surroundings are labeled in red.

- A
Structural Comparison of Ahp1‐Trx2 vs Ahp1‐Urm1 and close‐up.
- B
Superimposition of Ahp1‐Urm1 and Ahp1‐Trx2.

- A
Analysis of conjugation between ScUrm1‐SH with ScAhp1C31S (left) and CtAhp1C30S (right) using lysine‐less variants (ScAhp1C31S‐KtoR and CtAhp1C30S‐KtoR). ScUrm1 or CtUrm1C55S were used. The control reactions with Urm1‐OH and without TBH are indicated.
- B
Structural close‐up of the Ahp1‐Urm1 conjugation site with all nearby residues labeled and highlighted in balls‐and‐sticks representation (Cys60 in red). The last strand of the attached Urm1 protein (green) is shown in the cartoon. The distance between the peptide bond that forms between Lys63 (violet)/Gly111 (green) and Cys60 (red) is indicated.
- C
Analysis of conjugation between thiocarboxylated CtUrm1C55S and CtAhp1C30S variants, combining substitutions of serine and threonine residues in the active site and lysine‐less variants in the presence of TBH.
- D
MS/MS spectrum of the S‐ε‐GGH/T‐ε‐GGH containing peptide from CtAhp1C30S‐KtoR (top), and ScAhp1C31S‐KtoR (bottom) A representative annotated fragmentation spectrum is shown with the b‐ and y‐ions marked in red and blue, respectively. The peptide sequence is displayed at the top with the collision‐induced fragmentation pattern.
- E
Radioisotope assay for 35S‐labeled Urm1. Analysis of conjugation and sulfur transfer between Sc 35S‐Urm1 with ScAhp1 variants (left) and Ct 35S‐Urm1C55S with CtAhp1 variants in the presence (top) or absence of TBH (bottom). The samples were separated by nonreducing SDS–PAGE and dried gels were exposed overnight to storage phosphor screens. Uncropped images of the respective Coomassie‐stained gels before drying and the exposures themselves are provided in Appendix Fig S4D–G.
- F
(top) MS/MS spectrum of the persulfidated Cys60 and K63‐ε‐GGH containing peptide from CtAhp1C30S. (bottom) MS/MS spectrum of the persulfidated Cys62 peptide from ScAhp1C31S. Representative annotated fragmentation spectra are shown with the b‐ and y‐ions marked in red and blue, respectively. The peptide sequence is displayed at the top with the collision‐induced fragmentation pattern. Mass shift: +32 Da (persulfidation) or +89 Da (persulfidation + carbamidomethylation).
- G
Structural close‐up of the peroxidatic cysteine (Cys60) and Lys63 (violet) in CtAhp1C30S preconjugation (left) and unreacted Ahp1 from the conjugation reaction (right). The refined 2Fo‐Fc density (blue) and the Fo‐Fc omit map lacking the active site cysteine (green) is shown at comparable σ‐levels. For additional details see Appendix Fig S4.
- H
Same view as in G of the maps in the CtAhp1C30S‐CtUrm1C55S complex around Cys60 (red) at 3.5 σ (blue, left), 4 σ (green, left) and the anomalous difference Fourier map at 2 σ (violet, right). The respective resolution ranges of the maps in G and H are indicated.
- I
Proposed mechanism of urmylation. The peroxidatic cysteine of Ahp1 gets oxidized and sulfenylated. Urm1‐SH recognizes the sulfenylated cysteine and condenses to form an acyl disulfide intermediate. The formation of this intermediate can be reverted by reduction. A lysine/serine/threonine residue at proximal distance from the cysteine of the target protein mediates a nucleophilic attack on Urm1 to resolve the acyl disulfide. This results in a persulfidated cysteine and the covalent conjugation of the C‐terminus of Urm1 with the respective lysine/serine/threonine acceptor residue.

- A
Scatter plot of normalized SILAC H/L ratios (NEM‐treated versus untreated) for 547 quantified proteins, plotted against the sum of the respective peptide intensities. Proteins are color‐coded according to their respective P‐values (red < 0.001, orange 0.001–0.01, yellow 0.01–0.05 and blue > 0.05).
- B
Analysis of conjugation between thiocarboxylated ScUrm1 or HsUrm1 and various urmylation target proteins (ScAhp1C31S, HsPRDX5, ScTdh3, HsGAPDH, ScSes1, HsSARS1, ScPyk1, HsPKM2) in the presence of diamide. Unconjugated target proteins and Urm1 as well as their conjugates are indicated on the right.
- C
Schematic summary of the detected conjugated peptides by mass spectrometry for the various targets in (B). The thickness of the line represents the number of replicates where the respective site was detected, and the height represents the associated maximum Mascot ion score (n ≥ 3). The urmylation was identified as HisGlyGly tag on lysines, serines, and threonines, while persulfidation was searched as a mass shift: +32 Da (persulfidation) or +89 Da (persulfidation + carbamidomethylation) on cysteines.
- D
Analysis of conjugation between thiocarboxylated ScUrm1 or HsUrm1 and various urmylation target proteins (ScAhp1C31S, HsPRDX5, ScTdh3, HsGAPDH, ScSes1, HsSARS1, ScPyk1, HsPKM2) and their cysteine mutants in the presence of diamide. Unconjugated target proteins and Urm1 as well as their conjugates are indicated on the right.
- E
Radioisotope assay for 35S‐labeled Urm1. Analysis of conjugation and sulfur transfer between Sc 35S‐Urm1 with ScTdh3, ScSes1, and ScPyk1 in the presence or absence of diamide. The samples were separated by nonreducing SDS–PAGE and visualized by Coomassie staining. The dry gels were exposed overnight to storage phosphor screens. *Uba4 background.

- A
Analysis of covalent adduct formation by carboxylated (OH) or thiocarboxylated (SH) ScUbiquitin or ScSUMO on ScAhp1C31S or ScTdh3 in the presence of diamide. Thiocarboxylated ScUrm1 was used as a control. Unconjugated Ubls and target proteins as well as their conjugates are indicated on the right.
- B
Schematic summary of the conjugated peptides that were detected by mass spectrometry for ubiquitin or SUMO conjugation on Ahp1. The thickness of the line represents the number of replicates where the respective site was detected, and the height represents the associated ion score (n > 3). MS/MS spectrum of the ubiquitin (K‐ε‐GG/ S‐ε‐GG/ T‐ε‐GG) or SUMO (K‐ε‐GGIQ/ S‐ε‐GGIQ/ T‐ε‐GGIQ) conjugated peptide on ScAhp1C31S and the persulfidated cysteine spectrum by ubiquitin or SUMO. A representative annotated fragmentation spectrum is shown with b‐ and y‐ions marked in red and blue, respectively. The peptide sequence is shown at the top along with the collision‐induced fragmentation pattern. The ubiquitin or SUMO conjugation site was identified by detection of the GG and GGIQ remnant motif, respectively. m/z: mass to charge ratio. Persulfidation was searched as a mass shift: +32 Da (persulfidation) or +89 Da (persulfidation + carbamidomethylation) on cysteines.
- C
Analysis of covalent adduct formation between ScAhp1C31S and various thiocarboxylated C‐terminal variants of ScUrm1 in the presence of TBH. Unconjugated Urm1, Ahp1, and the conjugates are indicated on the right.

- A
TBH cytotoxicity assay in vivo. Basic growth and the synthetic effect in wild‐type ahp1Δ, urm1Δ, tum1Δ, ncs6Δ, cys3Δ, cys4Δ, cys3Δ urm1Δ, and cys4Δ urm1Δ strains was monitored without or with 1 mM TBH.
- B
H2S production using Biggy agar plates—Wild‐type, ahp1Δ, urm1Δ, tum1Δ, ncs6Δ, cys3Δ, cys4Δ, cys3Δ urm1Δ, and cys4Δ urm1Δ strains were grown on Biggy medium, and H2S production was recorded as indicated in the material and methods section.
- C
In vivo Ahp1 conjugation analyses with protein extracts obtained from the indicated yeast strains expressing HA‐URM1. Unconjugated Urm1 and Urm1‐Ahp1 conjugates were detected by anti‐HA (top panels) Western blots. Anti‐Ahp1 blots (middle) detect unmodified Ahp1. Anti‐Cdc19 (bottom panels) served as a loading control.
- D
Northern blot analysis for thiolation levels of in the genetic background of the indicated yeast strains. Total tRNA was resolved on denaturing PAGE supplemented with APM to retard the migration of thiolated . APM: ([N‐Acryloyl‐amino] phenyl) mercuric chloride. (bottom) Quantified thiolation levels from three independent biological replicates.
- E
Scheme of sulfur source for thiolation. Sulfur released as H2S from trans‐sulfuration enzymes Cys4 and Cys3 is used for protein persulfidation, scavenging free radicals metabolic signaling/stimulation. The sulfur from the Uba4‐Urm1 pathway is directed to tRNA thiolation and to the persulfidation of specific cysteine of target proteins.

- A
Analysis of in vitro conjugation between ScAhp1C31S or ScPyk1 and various thiocarboxylated variants of GFP in the presence of TBH. Unconjugated target proteins and the formed conjugates are indicated on the right.
- B
MS/MS Spectra of GFP conjugated to ScAhp1C31S via lysine. A representative annotated fragmentation spectrum is shown with the b‐ and y‐ions marked in red and blue, respectively. The peptide sequence is shown at the top with the collision‐induced fragmentation pattern. The GFP conjugation site was identified by the detection of conjugated lysine residues generated by chymotryptic digestion.
- C
ATP hydrolysis by ScUba4 WT in the presence of ScUrm1 variants, ScUbiquitin, ScSumo, and GFP variants. Data are representative of three independent experiments (n > 3; technical replicates). The error bars represent the calculated standard error of the mean (SEM).
- D
(Left) Crystal structure of Uba4 in complex with Urm1 (PDB ID 6YUC). The C‐terminus of Urm1 highlighted in red is conjugated to the catalytic Uba4C202K that is a mimetic of the thioester bond usually formed. (Right) Structural close‐up view showing the Uba4 active site with the residues of the Urm1 C‐terminus (red), the Uba4 the crossover loop (brown), the thioester intermediate (green), and the ATP molecule (transparent) in the nucleotide‐binding site.
- E
Analysis of in vitro conjugation of ScAhp1C31S, ScTdh3, and ScSes1 by carboxylated GFP‐Urm1 or Uba4‐thiocarboxylated GFP‐Urm1 in the presence or absence of diamide. Unconjugated proteins and the formed conjugates are indicated on the right.
Similar articles
-
The dual role of ubiquitin-like protein Urm1 as a protein modifier and sulfur carrier.Protein Cell. 2011 Aug;2(8):612-9. doi: 10.1007/s13238-011-1074-6. Epub 2011 Sep 9. Protein Cell. 2011. PMID: 21904977 Free PMC article. Review.
-
Sulfur transfer and activation by ubiquitin-like modifier system Uba4•Urm1 link protein urmylation and tRNA thiolation in yeast.Microb Cell. 2016 Oct 24;3(11):554-564. doi: 10.15698/mic2016.11.539. Microb Cell. 2016. PMID: 28357324 Free PMC article.
-
Molecular basis for the bifunctional Uba4-Urm1 sulfur-relay system in tRNA thiolation and ubiquitin-like conjugation.EMBO J. 2020 Oct 1;39(19):e105087. doi: 10.15252/embj.2020105087. Epub 2020 Sep 9. EMBO J. 2020. PMID: 32901956 Free PMC article.
-
Urm1: A Non-Canonical UBL.Biomolecules. 2021 Jan 22;11(2):139. doi: 10.3390/biom11020139. Biomolecules. 2021. PMID: 33499055 Free PMC article. Review.
-
Urmylation and tRNA thiolation functions of ubiquitin-like Uba4·Urm1 systems are conserved from yeast to man.FEBS Lett. 2015 Apr 2;589(8):904-9. doi: 10.1016/j.febslet.2015.02.024. Epub 2015 Mar 3. FEBS Lett. 2015. PMID: 25747390
Cited by
-
Molecular basis for thiocarboxylation and release of Urm1 by its E1-activating enzyme Uba4.Nucleic Acids Res. 2024 Dec 11;52(22):13980-13995. doi: 10.1093/nar/gkae1111. Nucleic Acids Res. 2024. PMID: 39673271 Free PMC article.
-
Chemical approaches to explore ubiquitin-like proteins.RSC Chem Biol. 2025 Feb 12;6(4):492-509. doi: 10.1039/d4cb00220b. eCollection 2025 Apr 2. RSC Chem Biol. 2025. PMID: 39950163 Free PMC article. Review.
-
Molecular choreography of E1 enzymes in ubiquitin-like protein cascades: New insights into dynamics and specificity.J Biol Chem. 2025 Jun 24;301(8):110415. doi: 10.1016/j.jbc.2025.110415. Online ahead of print. J Biol Chem. 2025. PMID: 40570956 Free PMC article. Review.
-
Encystation and Stress Responses under the Control of Ubiquitin-like Proteins in Pathogenic Amoebae.Microorganisms. 2023 Oct 31;11(11):2670. doi: 10.3390/microorganisms11112670. Microorganisms. 2023. PMID: 38004682 Free PMC article. Review.
-
In the moonlight: non-catalytic functions of ubiquitin and ubiquitin-like proteases.Front Mol Biosci. 2024 Feb 22;11:1349509. doi: 10.3389/fmolb.2024.1349509. eCollection 2024. Front Mol Biosci. 2024. PMID: 38455765 Free PMC article. Review.
References
-
- Bhogaraju S, Kalayil S, Liu Y, Bonn F, Colby T, Matic I, Dikic I (2016) Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell 167: 1636–1649 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions

