Prolonged, Low-Level Exposure to the Marine Toxin, Domoic Acid, and Measures of Neurotoxicity in Nonhuman Primates
- PMID: 36102641
- PMCID: PMC9472675
- DOI: 10.1289/EHP10923
Prolonged, Low-Level Exposure to the Marine Toxin, Domoic Acid, and Measures of Neurotoxicity in Nonhuman Primates
Abstract
Background: The excitotoxic molecule, domoic acid (DA), is a marine algal toxin known to induce overt hippocampal neurotoxicity. Recent experimental and epidemiological studies suggest adverse neurological effects at exposure levels near the current regulatory limit (20 ppm, ). At these levels, cognitive effects occur in the absence of acute symptoms or evidence of neuronal death.
Objectives: This study aimed to identify adverse effects on the nervous system from prolonged, dietary DA exposure in adult, female Macaca fascicularis monkeys.
Methods: Monkeys were orally exposed to 0, 0.075, and for an average of 14 months. Clinical blood counts, chemistry, and cytokine levels were analyzed in the blood. In-life magnetic resonance (MR) imaging assessed volumetric and tractography differences in and between the hippocampus and thalamus. Histology of neurons and glia in the fornix, fimbria, internal capsule, thalamus, and hippocampus was evaluated. Hippocampal RNA sequencing was used to identify differentially expressed genes. Enrichment of gene networks for neuronal health, excitotoxicity, inflammation/glia, and myelin were assessed with Gene Set Enrichment Analysis.
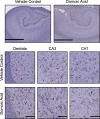
Results: Clinical blood counts, chemistry, and cytokine levels were not altered with DA exposure in nonhuman primates. Transcriptome analysis of the hippocampus yielded 748 differentially expressed genes (; ), reflecting differences in a broad molecular profile of intermediate early genes (e.g., FOS, EGR) and genes related to myelin networks in DA animals. Between exposed and control animals, MR imaging showed comparable connectivity of the hippocampus and thalamus and histology showed no evidence of hypomyelination. Histological examination of the thalamus showed a larger microglia soma size and an extension of cell processes, but suggestions of a response showed no indication of astrocyte hypertrophy.
Discussion: In the absence of overt hippocampal excitotoxicity, chronic exposure of Macaca fascicularis monkeys to environmentally relevant levels of DA suggested a subtle shift in the molecular profile of the hippocampus and the microglia phenotype in the thalamus that was possibly reflective of an adaptive response due to prolonged DA exposure. https://doi.org/10.1289/EHP10923.
Figures









Similar articles
-
Chronic, low-level oral exposure to marine toxin, domoic acid, alters whole brain morphometry in nonhuman primates.Neurotoxicology. 2019 May;72:114-124. doi: 10.1016/j.neuro.2019.02.016. Epub 2019 Feb 28. Neurotoxicology. 2019. PMID: 30826346 Free PMC article.
-
Power spectrum analysis of EEG in a translational nonhuman primate model after chronic exposure to low levels of the common marine neurotoxin, domoic acid.Neurotoxicology. 2020 Sep;80:124-129. doi: 10.1016/j.neuro.2020.07.006. Epub 2020 Jul 24. Neurotoxicology. 2020. PMID: 32717199 Free PMC article.
-
Effects of oral domoic acid exposure on maternal reproduction and infant birth characteristics in a preclinical nonhuman primate model.Neurotoxicol Teratol. 2019 Mar-Apr;72:10-21. doi: 10.1016/j.ntt.2019.01.001. Epub 2019 Jan 5. Neurotoxicol Teratol. 2019. PMID: 30615984 Free PMC article.
-
Domoic acid: neurobehavioral consequences of exposure to a prevalent marine biotoxin.Neurotoxicol Teratol. 2010 Mar-Apr;32(2):132-41. doi: 10.1016/j.ntt.2009.09.005. Epub 2009 Sep 30. Neurotoxicol Teratol. 2010. PMID: 19799996 Free PMC article. Review.
-
Domoic acid-induced neurotoxicity in the hippocampus of adult rats.Neurotox Res. 2004;6(2):105-17. doi: 10.1007/BF03033213. Neurotox Res. 2004. PMID: 15325963 Review.
Cited by
-
Toxicological and Pharmacological Activities, and Potential Medical Applications, of Marine Algal Toxins.Int J Mol Sci. 2024 Aug 24;25(17):9194. doi: 10.3390/ijms25179194. Int J Mol Sci. 2024. PMID: 39273145 Free PMC article. Review.
-
Transcriptomics as an Early Warning of Domoic Acid Exposure in Pacific Razor Clams (Siliqua patula).Toxins (Basel). 2025 Apr 11;17(4):194. doi: 10.3390/toxins17040194. Toxins (Basel). 2025. PMID: 40278693 Free PMC article.
-
Effects of marine biotoxins on drug-metabolizing cytochrome P450 enzymes and their regulation in mammalian cells.Arch Toxicol. 2024 May;98(5):1311-1322. doi: 10.1007/s00204-024-03694-6. Epub 2024 Feb 28. Arch Toxicol. 2024. PMID: 38416141 Free PMC article. Review.
-
Invited Perspective: The Relevance of Animal Models of Domoic Acid Neurotoxicity to Human Health.Environ Health Perspect. 2022 Sep;130(9):91302. doi: 10.1289/EHP11774. Epub 2022 Sep 14. Environ Health Perspect. 2022. PMID: 36102794 Free PMC article. No abstract available.
-
The Toxic Effects of Environmental Domoic Acid Exposure on Humans and Marine Wildlife.Mar Drugs. 2025 Jan 29;23(2):61. doi: 10.3390/md23020061. Mar Drugs. 2025. PMID: 39997185 Free PMC article. Review.
References
-
- Steele TS, Brunson JK, Maeno Y, Terada R, Allen AE, Yotsu-Yamashita M, et al. . 2022. Domoic acid biosynthesis in the red alga Chondria armata suggests a complex evolutionary history for toxin production. Proc Natl Acad Sci USA 119(6):e2117407119, PMID: , 10.1073/pnas.2117407119. - DOI - PMC - PubMed
-
- Trainer VL, Bates SS, Lundholm N, Thessen AE, Cochlan WP, Adams NG, et al. . 2012. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 14:271–300, 10.1016/j.hal.2011.10.025. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

