Modulation of SOCS3 Levels via STAT3 and Estrogen-ERαp66 Signaling during Hepatitis E Virus Replication in Hepatocellular Carcinoma Cells
- PMID: 36102649
- PMCID: PMC9555149
- DOI: 10.1128/jvi.01008-22
Modulation of SOCS3 Levels via STAT3 and Estrogen-ERαp66 Signaling during Hepatitis E Virus Replication in Hepatocellular Carcinoma Cells
Abstract
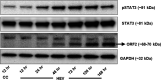
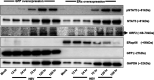
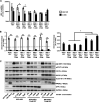
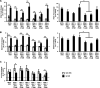
Hepatitis E virus (HEV) infection usually results in a self-limiting acute disease; however, in infected pregnant women, it is associated with increased mortality and fulminant hepatic failure. Estrogen is known to be elevated during pregnancy, and estrogen signaling via classical estrogen receptor-ERα is known to regulate hepatocyte function and host innate immune response, including the STAT3 pathway. In this study, we investigated whether the estrogen classical signaling pathway via ERαp66 has any effect on STAT3 activation during HEV replication and HEV-induced IFN response. We first demonstrated that Huh7-S10-3 liver cells expressed the nonfunctional estrogen receptor ERαp36 isoform and lack the functional ERαp66 isoform. We further showed persistent phosphorylated-STAT3 levels in genotype 3 human HEV (Kernow P6 strain) RNA-transfected cells at later time points. In Huh7-S10-3 cells, estrogen at first-to-third trimester concentration (7.3 to 73 nM) did not significantly affect HEV replication; however, blocking of STAT3 activation led to a decrease in the HEV ORF2 protein level. Our mechanistic study revealed that STAT3 differentially regulates SOCS3 and type-III interferon (IFN) levels during HEV replication and the presence of estrogen-ERαp66 signaling stabilizes SOCS3 levels in vitro. We also demonstrate that HEV infection in pregnant and nonpregnant rabbits led to a significant increase in IFN response as measured by increased levels of IFN-stimulated-gene-15 (ISG15) mRNA levels irrespective of pregnancy status. Collectively, the results indicate that estrogen signaling and STAT3 regulate SOCS3 and IFN responses in vitro during HEV replication. The results have important implications for understanding HEV replication and HEV-induced innate immune response in pregnant women. IMPORTANCE Hepatitis E is usually a self-resolving acute disease; however, in pregnant women, HEV infection is associated with high mortality and fulminant hepatic failure. During pregnancy, estrogen levels are elevated, and in the liver, the estrogen receptor ERα is predominant and estrogen signaling is known to regulate hepatocyte metabolism and leptin-induced STAT3 levels. Viruses can module host innate immune response via STAT3. Therefore, in this study, we investigated whether STAT3 and estrogen-classical signaling via the ERαp66 pathway modulate HEV replication and HEV-induced innate immune response. We demonstrated that estrogen signaling did not affect HEV replication in human liver cells, but blocking of STAT3 activation reduced HEV capsid protein levels in human liver cells. We also showed that inhibition of STAT3 activation reduced SOCS3 levels, while the presence of the estrogen-ERαp66 signaling pathway stabilized SOCS3 levels. The results from this study will aid our understanding of the mechanism of HEV pathogenesis and immune response during pregnancy.
Keywords: SOCS3; STAT3; estrogen; genotype 3 HEV; hepatitis E virus (HEV); pregnancy; replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Progesterone-Mediated Enhancement of Hepatitis E Virus Replication in Human Liver Cells.mBio. 2021 Jun 29;12(3):e0143421. doi: 10.1128/mBio.01434-21. Epub 2021 Jun 22. mBio. 2021. PMID: 34154410 Free PMC article.
-
ISG15 Modulates Type I Interferon Signaling and the Antiviral Response during Hepatitis E Virus Replication.J Virol. 2017 Sep 12;91(19):e00621-17. doi: 10.1128/JVI.00621-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28724761 Free PMC article.
-
Innate immune responses in human hepatocyte-derived cell lines alter genotype 1 hepatitis E virus replication efficiencies.Sci Rep. 2016 May 27;6:26827. doi: 10.1038/srep26827. Sci Rep. 2016. PMID: 27230536 Free PMC article.
-
Interplay between Hepatitis E Virus and Host Cell Pattern Recognition Receptors.Int J Mol Sci. 2021 Aug 26;22(17):9259. doi: 10.3390/ijms22179259. Int J Mol Sci. 2021. PMID: 34502167 Free PMC article. Review.
-
Advances in Hepatitis E Virus Biology and Pathogenesis.Viruses. 2021 Feb 9;13(2):267. doi: 10.3390/v13020267. Viruses. 2021. PMID: 33572257 Free PMC article. Review.
Cited by
-
Impact of PEDV infection on the biological characteristics of porcine intestinal exosomes.Front Microbiol. 2024 May 13;15:1392450. doi: 10.3389/fmicb.2024.1392450. eCollection 2024. Front Microbiol. 2024. PMID: 38803376 Free PMC article.
-
Fetal Loss in Pregnant Rabbits Infected with Genotype 3 Hepatitis E Virus Is Associated with Altered Inflammatory Responses, Enhanced Virus Replication, and Extrahepatic Virus Dissemination with Positive Correlations with Increased Estradiol Level.mBio. 2023 Apr 25;14(2):e0041823. doi: 10.1128/mbio.00418-23. Epub 2023 Mar 20. mBio. 2023. PMID: 36939322 Free PMC article.
-
Estrogen and viral infection.Front Immunol. 2025 May 16;16:1556728. doi: 10.3389/fimmu.2025.1556728. eCollection 2025. Front Immunol. 2025. PMID: 40453078 Free PMC article. Review.
-
Double-edged sword of JAK/STAT signaling pathway in viral infections: novel insights into virotherapy.Cell Commun Signal. 2023 Oct 2;21(1):272. doi: 10.1186/s12964-023-01240-y. Cell Commun Signal. 2023. PMID: 37784164 Free PMC article. Review.
-
Sex disparities in hepatocellular carcinoma immunotherapy: hormonal and genetic influences on treatment efficacy.Front Immunol. 2025 May 14;16:1607374. doi: 10.3389/fimmu.2025.1607374. eCollection 2025. Front Immunol. 2025. PMID: 40438106 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

