Active Pharmaceutical Ingredient Uptake by Zebrafish (Danio rerio) Oct2 (slc22a2) Transporter Expressed in Xenopus laevis Oocytes
- PMID: 36102855
- PMCID: PMC9827845
- DOI: 10.1002/etc.5480
Active Pharmaceutical Ingredient Uptake by Zebrafish (Danio rerio) Oct2 (slc22a2) Transporter Expressed in Xenopus laevis Oocytes
Abstract
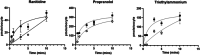
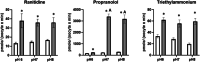
Uptake of active pharmaceutical ingredients (APIs) across the gill epithelium of fish is via either a passive or facilitated transport process, with the latter being more important at the lower concentrations more readily observed in the environment. The solute carrier (SLC) 22A family, which includes the organic cation transporter OCT2 (SLC22A2), has been shown in mammals to transport several endogenous chemicals and APIs. Zebrafish oct2 was expressed in Xenopus oocytes and the uptake of ranitidine, propranolol, and tetraethylammonium characterized. Uptake of ranitidine and propranolol was time- and concentration-dependent with a km and Vmax for ranitidine of 246 µM and 45 pmol/(oocyte × min) and for propranolol of 409 µM and 190 pmol/(oocyte × min), respectively. Uptake of tetraethylammonium (TEA) was inhibited by propranolol, amantadine, and cimetidine, known to be human OCT2 substrates, but not quinidine or ranitidine. At external media pH 7 and 8 propranolol uptake was 100-fold greater than at pH 6; pH did not affect ranitidine or TEA uptake. It is likely that cation uptake is driven by the electrochemical gradient across the oocyte. Uptake kinetics parameters, such as those derived in the present study, coupled with knowledge of transporter localization and abundance and API metabolism, can help derive pharmacokinetic models. Environ Toxicol Chem 2022;41:2993-2998. © 2022 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Keywords: Absorption; emerging pollutants; in vitro toxicology; pharmaceuticals.
© 2022 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Conflict of interest statement
Elisabeth D. Chang, Christer Hogstrand, and Nic R. Bury declare no conflict of interest.
Figures


References
-
- Arndt, P. , Volk, C. , Gorboulev, V. , Budiman, Y. , Popp, C. , Ulzheimer‐Teuber, I. , Akhoudova, A. , Koppatz, S. , Bamberg, E. , Nagel, G. , & Koepsell, H. (2001). Interaction of cations, anions, and weak base quinine with rat renal cation transporter rOCT2 compared with rOCT1. American Journal of Physiology, 281, F454–F468. - PubMed
-
- Avdeef, A. , Box, K. J. , Comer, J. E. A. , Hibbert, C. , & Tam, K. Y. (1998). pH‐Metric logP10. Determination of liposomal membrane‐water partition coefficients of ionizable drugs. Pharmaceutical Research, 5, 209–215. - PubMed
-
- Bourdet, D. L. , Pritchard, J. B. , & Thakker, D. R. (2005). Differential substrate and inhibitory activities of raitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC 22A2, and hOCT3 (SLC22A3). Journal of Pharmacology and Experimental Therapeutics, 315, 1288–1297. - PubMed
-
- Bourdet, D. L. , & Thakker, D. R. (2006). Saturable absorptive transport of the hydrophilic organic cation ranitidine in Caco‐2 cells: Role of pH‐dependent organic cation uptake system and P‐Glycoprotein. Pharmaceutical Research, 23, 1165–1177. - PubMed
-
- Busch, A. E. , Karbach, U. , Miska, D. , Gorboulev, V. , Akhoundova, A. , Volk, C. , Arndt, P. , Ulzheimer, J. C. , Sonders, M. S. , Baumann, C. , Waldegger, S. , Lang, F. , & Koepsell, H. (1998). Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Molecular Pharmacology, 54, 342–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

