Mitochondrial dysfunction triggers actin polymerization necessary for rapid glycolytic activation
- PMID: 36102863
- PMCID: PMC9477750
- DOI: 10.1083/jcb.202201160
Mitochondrial dysfunction triggers actin polymerization necessary for rapid glycolytic activation
Abstract
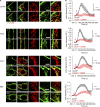
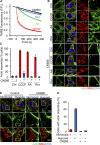
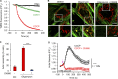
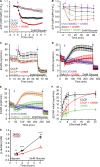
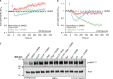
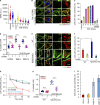
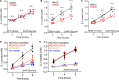
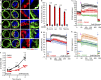
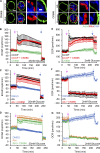
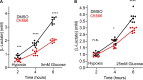
Mitochondrial damage represents a dramatic change in cellular homeostasis. One rapid response is perimitochondrial actin polymerization, termed acute damage-induced actin (ADA). The consequences of ADA are not understood. In this study, we show evidence suggesting that ADA is linked to rapid glycolytic activation upon mitochondrial damage in multiple cells, including mouse embryonic fibroblasts and effector CD8+ T lymphocytes. ADA-inducing treatments include CCCP, antimycin, rotenone, oligomycin, and hypoxia. The Arp2/3 complex inhibitor CK666 or the mitochondrial sodium-calcium exchanger (NCLX) inhibitor CGP37157 inhibits both ADA and the glycolytic increase within 5 min, supporting ADA's role in glycolytic stimulation. Two situations causing chronic reductions in mitochondrial ATP production, mitochondrial DNA depletion and mutation to the NDUFS4 subunit of complex 1 of the electron transport chain, cause persistent perimitochondrial actin filaments similar to ADA. CK666 treatment causes rapid mitochondrial actin loss and a drop in ATP in NDUFS4 knock-out cells. We propose that ADA is necessary for rapid glycolytic activation upon mitochondrial impairment, to re-establish ATP production.
© 2022 Chakrabarti et al.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

