Effect of Different Itraconazole Dosing Regimens on Cure Rates, Treatment Duration, Safety, and Relapse Rates in Adult Patients With Tinea Corporis/Cruris: A Randomized Clinical Trial
- PMID: 36103158
- PMCID: PMC9475442
- DOI: 10.1001/jamadermatol.2022.3745
Effect of Different Itraconazole Dosing Regimens on Cure Rates, Treatment Duration, Safety, and Relapse Rates in Adult Patients With Tinea Corporis/Cruris: A Randomized Clinical Trial
Abstract
Importance: With worldwide emergence of recalcitrant and resistant dermatophytosis, itraconazole is increasingly being used as the first-line drug for treatment of tinea corporis/cruris (TCC). Apparent inadequacy with low doses has led to empirical use of higher doses and antifungal combinations.
Objective: To compare cure rates, treatment durations, safety profiles, and relapse rates of itraconazole 100, 200, and 400 mg/d for the treatment of TCC.
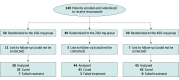
Design, setting, and participants: This double-blind randomized clinical trial included adult patients with treatment-naive TCC involving at least 5% body surface area. Patients were recruited from the dermatology outpatient department of a tertiary care hospital in New Delhi, India between March 1, 2020, and August 31, 2021.
Interventions: Patients were randomized to 1 of the 3 treatment groups. Biweekly blinded assessments were performed until cure or treatment failure. Posttreatment follow-up of at least 8 weeks was conducted to detect relapses.
Main outcome and measures: Cure rates, treatment durations, safety profiles, and relapse rates were assessed. Secondary outcomes included comparison of rapidity of clinical response and cost-effectiveness between groups.
Results: Of the 149 patients assessed, the mean (SD) age was 34.3 (12.2) years, 69 patients (46.4%) were women, and 80 patients (53.6%) were men. The difference in cure rate between the 100- and 200-mg groups was statistically nonsignificant (hazard ratio [HR], 1.44; 95% CI, 0.91-2.30; P = .12), while the difference between the 100- and 400-mg groups (HR, 2.87; 95% CI, 1.78-4.62; P < .001) and between the 200- and 400-mg groups (HR, 1.99; 95% CI, 1.28-3.09; P = .002) was statistically significant. Mean (SD) treatment durations were statistically significantly different between the 100- and 400-mg groups (7.7 [4.7] weeks vs 5.2 [2.6] weeks; P = .03) and between the 200- and 400-mg groups (7.2 [3.8] weeks vs 5.2 [2.6] weeks; P = .004), but the difference between the 100- and 200-mg groups was not statistically significant. A total of 55 patients (47.4%) relapsed after treatment. Relapse rates were comparable across groups. No patient discontinued treatment due to adverse effects. Treatment with the 200-mg dose incurred a 63% higher cost and 400 mg a 120% higher cost over 100 mg in achieving cure.
Conclusions and relevance: In this randomized clinical trial, high overall efficacy was observed among the 3 itraconazole doses for treatment of TCC, but with prolonged treatment durations and considerable relapse rates. Treatment with the 200- and 100-mg doses did not differ significantly in efficacy or treatment durations, while 400 mg scored over the other 2 on these outcomes. Considerable additional cost is incurred in achieving cure with the 200- and 400-mg doses.
Trial registration: Clinical Trials Registry of India Identifier: CTRI/2020/03/024326.
Conflict of interest statement
Figures
Comment in
- doi: 10.1001/jamadermatol.2022.3736
References
-
- Khurana A, Masih A, Chowdhary A, et al. . Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother. 2018;62(12):e01038-e18. doi:10.1128/AAC.01038-18 - DOI - PMC - PubMed
-
- Singh A, Masih A, Monroy-Nieto J, et al. . A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: genomic insights and resistance profile. Fungal Genet Biol. 2019;133:103266. doi:10.1016/j.fgb.2019.103266 - DOI - PubMed