Effect of Minocycline on Depressive Symptoms in Patients With Treatment-Resistant Depression: A Randomized Clinical Trial
- PMID: 36103181
- PMCID: PMC9475381
- DOI: 10.1001/jamanetworkopen.2022.30367
Effect of Minocycline on Depressive Symptoms in Patients With Treatment-Resistant Depression: A Randomized Clinical Trial
Abstract
Importance: Insufficient treatment response and resulting chronicity constitute a major problem in depressive disorders. Remission rates range as low as 15% to 40% and treatment-resistant depression (TRD) is associated with low-grade inflammation, suggesting anti-inflammatory interventions as a rational treatment strategy. Minocycline, which inhibits microglial activation, represents a promising repurposing candidate in the treatment of TRD.
Objective: To determine whether 6 weeks of minocycline as add-on to antidepressant treatment as usual can significantly reduce depressive symptoms in patients with TRD.
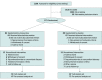
Design, setting, and participants: The study was conducted in Germany and designed as a multicenter double-blind randomized clinical trial (RCT) of 200 mg/d minocycline treatment over a course of 6 weeks with a 6-month follow-up. Participants were recruited from January 2016 to August 2020 at 9 university hospitals that served as study sites. Key inclusion criteria were a diagnosis of major depressive disorder (according to Diagnostic and Statistical Manual of Mental Disorders [Fifth Edition] criteria), severity of depressive symptoms on the Hamilton Depression Rating Scale (HAMD-17) greater than or equal to 16 points, aged 18 to 75 years, body mass index 18 to 40, Clinical Global Impression Scale (CGI-S) greater than or equal to 4, failure to adequately respond to an initial antidepressant standard medication as per Massachusetts General Hospital Antidepressant Treatment History Questionnaire, and stable medication for at least 2 weeks. A total of 258 patients were screened, of whom 173 were randomized and 168 were included into the intention-to-treat population. Statistical analysis was performed from April to November 2020.
Interventions: Participants were randomized (1:1) to receive adjunct minocycline (200 mg/d) or placebo for 6 weeks.
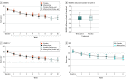
Main outcomes and measures: Primary outcome measure was the change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline to week 6 analyzed by intention-to-treat mixed model repeated measures. Secondary outcome measures were response, remission, and various other clinical rating scales.
Results: Of 173 eligible and randomized participants (84 randomized to minocycline and 89 randomized to placebo), 168 formed the intention-to-treat sample (79 [47.0%] were women, 89 [53.0%] were men, 159 [94.6%] were White, 9 [6.4%] were of other race and ethnicity, including Asian and unknown ethnicity), with 81 in the minocycline group and 87 in the placebo group. The mean (SD) age was 46.1 (13.1) years, and the mean (SD) MADRS score at baseline was 26.5 (5.0). There was no difference in rates of completion between the minocycline (83.3% [70 of 81]) and the placebo group (83.1% [74 of 87]). Minocycline treatment did not alter the course of depression severity compared with placebo as assessed by a decrease in MADRS scores over 6 weeks of treatment (1.46 [-1.04 to 3.96], P = .25). Minocycline treatment also exhibited no statistically significant effect on secondary outcomes.
Conclusions and relevance: In this large randomized clinical trial with minocycline at a dose of 200 mg/d added to antidepressant treatment as usual for 6 weeks, minocycline was well tolerated but not superior to placebo in reducing depressive symptoms in patients with TRD. The results of this RCT emphasize the unmet need for therapeutic approaches and predictive biomarkers in TRD.
Trial registration: EU Clinical Trials Register Number: EudraCT 2015-001456-29.
Conflict of interest statement
Figures
References
-
- James SL, Abate D, Abate KH, et al. . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1789-1858. doi:10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

