Protocol to measure endotoxin from opaque tissues in mice using an optimized kinetic limulus amebocyte lysate assay
- PMID: 36103303
- PMCID: PMC9483641
- DOI: 10.1016/j.xpro.2022.101669
Protocol to measure endotoxin from opaque tissues in mice using an optimized kinetic limulus amebocyte lysate assay
Abstract
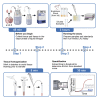
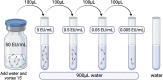
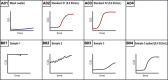
Endotoxin accumulation has been widely noted in several pathologies ranging from metabolic dysregulation to bacterial infection. Using limulus amebocyte lysate (LAL) assays to detect endotoxin load has been the only reliable way to assess endotoxin accumulation, but assays optimized for detection in opaque tissues are still lacking. We optimized a sensitive Kinetic LAL assay for endotoxin detection from murine tissues. In this protocol, we describe tissue collection and homogenization, followed by the procedure to run the assay and data analysis. For complete details on the use and execution of this protocol, please refer to Ceasrine et al. (2022).
Keywords: Immunology; Neuroscience.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Detection of endotoxin in biological products by the limulus test.Dev Biol Stand. 1977;34:7-13. Dev Biol Stand. 1977. PMID: 838151
-
[Content detection of bacterial endotoxin in two kinds of injection by gelatin technique].Zhongguo Zhong Yao Za Zhi. 2010 Jun;35(11):1405-9. Zhongguo Zhong Yao Za Zhi. 2010. PMID: 20822008 Chinese.
-
Sensitive quantitation of endotoxin by enzyme-linked immunosorbent assay with monoclonal antibody against Limulus peptide C.J Clin Microbiol. 1994 Feb;32(2):416-22. doi: 10.1128/jcm.32.2.416-422.1994. J Clin Microbiol. 1994. PMID: 8150951 Free PMC article.
-
Currently Available Recombinant Alternatives to Horseshoe Crab Blood Lysates: Are They Comparable for the Detection of Environmental Bacterial Endotoxins? A Review.PDA J Pharm Sci Technol. 2020 Sep-Oct;74(5):602-611. doi: 10.5731/pdajpst.2020.012187. Epub 2020 Aug 14. PDA J Pharm Sci Technol. 2020. PMID: 32817324 Review.
-
[Application of a bacterial endotoxin test for parenteral drugs].Eisei Shikenjo Hokoku. 1994;(112):209-11. Eisei Shikenjo Hokoku. 1994. PMID: 8854936 Review. Japanese.
Cited by
-
Gonadal hormones impart male-biased behavioral vulnerabilities to immune activation via microglial mitochondrial function.Brain Behav Immun. 2024 Jan;115:680-695. doi: 10.1016/j.bbi.2023.11.010. Epub 2023 Nov 14. Brain Behav Immun. 2024. PMID: 37972878 Free PMC article.
References
-
- Cooper J.F., Weary M.E., Jordan F.T. The impact of non–endotoxin LAL–reactive materials on limulus amebocyte lysate analyses. PDA J. Pharm. Sci. Technol. 1997;51:2–6. - PubMed
-
- Sandle T. A comparative study of different methods for endotoxin destruction. 2013. http://www.americanpharmaceuticalreview.com/Featured-Articles/148858-A-C...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

