Fluvoxamine for the treatment of COVID-19
- PMID: 36103313
- PMCID: PMC9473347
- DOI: 10.1002/14651858.CD015391
Fluvoxamine for the treatment of COVID-19
Abstract
Background: Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) that has been approved for the treatment of depression, obsessive-compulsive disorder, and a variety of anxiety disorders; it is available as an oral preparation. Fluvoxamine has not been approved for the treatment of infections, but has been used in the early treatment of people with mild to moderate COVID-19. As there are only a few effective therapies for people with COVID-19 in the community, a thorough understanding of the current evidence regarding the efficacy and safety of fluvoxamine as an anti-inflammatory and possible anti-viral treatment for COVID-19, based on randomised controlled trials (RCTs), is needed.
Objectives: To assess the efficacy and safety of fluvoxamine in addition to standard care, compared to standard care (alone or with placebo), or any other active pharmacological comparator with proven efficacy for the treatment of COVID-19 outpatients and inpatients.
Search methods: We searched the Cochrane COVID-19 Study Register (including Cochrane Central Register of Controlled Trials, MEDLINE, Embase, ClinicalTrials.gov, WHO ICTRP, medRxiv), Web of Science and WHO COVID-19 Global literature on COVID-19 to identify completed and ongoing studies up to 1 February 2022.
Selection criteria: We included RCTs that compared fluvoxamine in addition to standard care (also including no intervention), with standard care (alone or with placebo), or any other active pharmacological comparator with proven efficacy in clinical trials for the treatment of people with confirmed COVID-19, irrespective of disease severity, in both inpatients and outpatients. Co-interventions needed to be the same in both study arms. We excluded studies comparing fluvoxamine to other pharmacological interventions with unproven efficacy.
Data collection and analysis: We assessed risk of bias of primary outcomes using the Cochrane Risk of Bias 2 tool for RCTs. We used GRADE to rate the certainty of evidence to treat people with asymptomatic to severe COVID-19 for the primary outcomes including mortality, clinical deterioration, clinical improvement, quality of life, serious adverse events, adverse events of any grade, and suicide or suicide attempt.
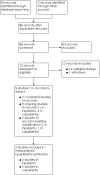
Main results: We identified two completed studies with a total of 1649 symptomatic participants. One study was conducted in the USA (study with 152 participants, 80 and 72 participants per study arm) and the other study in Brazil (study with 1497 high-risk participants for progression to severe disease, 741 and 756 participants per study arm) among outpatients with mild COVID-19. Both studies were double-blind, placebo-controlled trials in which participants were prescribed 100 mg fluvoxamine two or three times daily for a maximum of 15 days. We identified five ongoing studies and two studies awaiting classification (due to translation issues, and due to missing published data). We found no published studies comparing fluvoxamine to other pharmacological interventions of proven efficacy. We assessed both included studies to have an overall high risk of bias. Fluvoxamine for the treatment of COVID-19 in inpatients We did not identify any completed studies of inpatients. Fluvoxamine for the treatment of COVID-19 in outpatients Fluvoxamine in addition to standard care may slightly reduce all-cause mortality at day 28 (RR 0.69, 95% CI 0.38 to 1.27; risk difference (RD) 9 per 1000; 2 studies, 1649 participants; low-certainty evidence), and may reduce clinical deterioration defined as all-cause hospital admission or death before hospital admission (RR 0.55, 95% CI 0.16 to 1.89; RD 57 per 1000; 2 studies, 1649 participants; low-certainty evidence). We are very uncertain regarding the effect of fluvoxamine on serious adverse events (RR 0.56, 95% CI 0.15 to 2.03; RD 54 per 1000; 2 studies, 1649 participants; very low-certainty evidence) or adverse events of any grade (RR 1.06, 95% CI 0.82 to 1.37; RD 7 per 1000; 2 studies, 1649 participants; very low-certainty evidence). Neither of the studies reported on symptom resolution (clinical improvement), quality of life or suicide/suicide attempt.
Authors' conclusions: Based on a low-certainty evidence, fluvoxamine may slightly reduce all-cause mortality at day 28, and may reduce the risk of admission to hospital or death in outpatients with mild COVID-19. However, we are very uncertain regarding the effect of fluvoxamine on serious adverse events, or any adverse events. In accordance with the living approach of this review, we will continually update our search and include eligible trials as they arise, to complete any gaps in the evidence.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
John Nyirenda (JN): has no known conflicts of interest to declare.
Mario Sofroniou (MSo): has no known conflicts of interest to declare.
Ingrid Toews (IT): has no known conflicts of interest to declare.
Agata Mikolajewska (AMi): is affiliated with not‐for‐profit organisation: Coordination of Section COVRIIN and Work in Office of STAKOB (Competence and Treatment Centres for high consequence infectious diseases) at Robert Koch Institute Centre for Biological Threats and Special Pathogens (ZBS), Section Clinical Management and Infection Control.
Cornelius Lehane (CL): has no known conflicts of interest to declare.
Ina Monsef (IM): has no known conflicts of interest to declare. She is part of the Cochrane Haematology editorial team, but was not involved in the editorial process of this review.
Aesha Abu‐taha (AA): has no known conflicts of interest to declare.
Andy Maun (AMa) is a member of the guideline group 'Covid 19, Ambulatory Treatment', member of the German College of General Practitioners and Family Physicians, and affiliated with the Practice Kreusel/Höltner, Titisee‐Neustadt, Germany (employed as a General Practitioner).
Miriam Stegemann (MSt): has no known conflicts of interest to declare.
Christine Schmucker (CS): has no known conflicts of interest to declare.
Figures




References
References to studies included in this review
Lenze 2020 {published data only}
-
- NCT04342663. A double-blind, placebo-controlled clinical trial of fluvoxamine for symptomatic individuals with COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04342663 (first received 13 April 2020).
TOGETHER 2021 {published data only}
-
- NCT04727424. Repurposed approved and under development therapies for patients with early-onset COVID-19 and mild symptoms. www.clinicaltrials.gov/ct2/show/NCT04727424 (first received 27 January 2021).
-
- Reis G, Dos Santos Moreira-Silva EA, Silva DC, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Global Health 2021;10(1):e42-e51. [DOI: 10.1016/S2214-109X(21)00448-4] - DOI - PMC - PubMed
References to studies excluded from this review
Assanovich 2021 {published data only}
-
- Assanovich M. Fluvoxamine in the treatment of patients with COVID-19. Psychiatry, Psychotherapy and Clinical Psychology 2021;12(2):260-8.
Brown University 2021 {published data only}
-
- Anonymous. Study suggests fluvoxamine may limit deterioration from COVID‐19. Brown University Psychopharmacology Update 2021;32(4):6-7.
Glebov 2021 {published data only}
Hashimoto 2021 {unpublished data only}
Khosravi 2022 {published data only}
Marcec 2021 {published data only}
McCarthy 2021 {published data only}
-
- McCarthy MW. Repurposing approach of fluvoxamine for COVID-19. Drug Future 2021;46(10):813-8.
Medical Letter 2021 {published data only}
-
- Anonymous. Fluvoxamine for COVID-19? Medical Letter on Drugs and Therapeutics 2021;63(1623):69-70. - PubMed
Murchu 2021 {published data only}
NCT04711863 {unpublished data only}
-
- NCT04711863. Fluvoxamine for adults with mild to moderate COVID-19 [Fluvoxamine for adults with mild to moderate COVID-19: a single-blind, randomized, placebo-controlled trial]. clinicaltrials.gov/ct2/show/record/NCT04711863 (first received 15 January 2021).
Seftel 2021 {published data only}
References to studies awaiting assessment
NCT04668950 {unpublished data only}
-
- NCT04668950. Fluvoxamine for early treatment of Covid-19 (Stop Covid 2). clinicaltrials.gov/ct2/show/NCT04668950 (first received 16 December 2020).
Safa 2020 {published and unpublished data}
-
- Safa M, Hashemian MR, Abedi M, Malek MM. Effect of fluvoxamine medicine on cytokine level of COVID-19 patients, hospitalized in ICU ward [أثیر داروی فلووکسامین بر سطح سیتوکین بیماران مبتال به9-COVID بستری در بخش ICU]. Journal of Medical Council of Iran 2020;38(3):135-8. [URL: jmciri.ir/browse.php?a_code=A-10-1-2643&sid=1&slc_lang=en]
References to ongoing studies
NCT04510194 {unpublished data only}
-
- NCT04510194. COVID-OUT: early outpatient treatment for SARS-CoV-2 infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04510194. (first received 12 August 2020).
NCT04718480 {unpublished data only}
-
- NCT04718480. Fluvoxamine administration in moderate SARS-CoV-2 (COVID-19) infected patients. clinicaltrials.gov/ct2/show/record/NCT04718480 (first received 22 January 2021).
NCT04885530 {unpublished data only}
-
- NCT04885530. ACTIV-6: COVID-19 study of repurposed medications. clinicaltrials.gov/ct2/show/NCT04885530 (first received on 13 May 2021).
NCT05087381 {unpublished data only}
-
- NCT05087381. Randomized-controlled trial of the effectiveness of COVID-19 early treatment in community. clinicaltrials.gov/ct2/show/record/NCT05087381 (first received 21 October 2021).
TCTR20210615002 {unpublished data only}
-
- TCTR20210615002. Effect of combined fluvoxamine with favipiravir versus favipiravir monotherapy in prevention of clinical deterioration among mild to moderate COVID-19 patients monitoring by telemedicine in virtual clinic: open-label randomized controlled trial. www.thaiclinicaltrials.org/show/TCTR20210615002 (first received 15 June 2021).
Additional references
Agarwal 2020
Altmann 2021
Ansems 2021
Beigel 2020
Buitrago‐Garcia 2020
-
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci Aziz M, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2infections: a living systematic review and meta-analysis. PloS Medicine 2020;17(9):e1003346. [DOI: 10.1371/ journal.pmed.1003346] - PMC - PubMed
Chen 2020
COMET 2021
-
- COMET Initiative. Core outcome set developers’ response to COVID-19; April 2021. www.comet-initiative.org/Studies/Details/1538.
Danza 2022
-
- Danza P, Koo TH, Haddix M, Fisher R, Traub E, Yong K, et al. SARS-CoV-2 infection and hospitalization among adults aged≥ 18 Years, by vaccination status, before and during SARS-CoV-2 B. 1.1. 529 (omicron) variant predominance—Los Angeles County, California, November 7, 2021 to January 8, 2022. Morbidity and Mortality Weekly Report 2022;71(5):177. [DOI: 10.15585/mmwr.mm7105e1] - DOI - PMC - PubMed
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
DEGAM 2022
-
- Blankenfeld H, Kaduszkiewicz H, Kochen MM, Pömsl J, Scherer M. SARS-CoV-2/Covid-19information and practical aids for established general practitioners and general practitioners (Version 22) [SARS-CoV-2/ Covid-19-Informationen & Praxishilfen für niedergelassene Hausärztinnen und Hausärzte (Version 22)]. www.degam-leitlinien.de/ (accessed 16 June 2022).
Egger 1997
Figgit 2000
-
- Figgitt DP, McClellan KJ. Fluvoxamine. An updated review of its use in the management of adults with anxiety disorders. SpringerLink 2000;60(4):925-54. [PMID: ] - PubMed
Friedman 2014
Funk 2021
-
- Funk T, Pharris A, Spiteri G, Bundle N, Melidou A, Carr M, et al. Characteristics of SARS-CoV-2 variants of concern B.1.1.7,B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Europe's Journal on Infectious Disease Surveillance, Epidemiology, Prevention and Control 2021;26(16):pii=2100348. [DOI: 10.2807/1560-7917.ES.2021.26.16.2100348] - DOI - PMC - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 15 August 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grubaugh 2020
Gupta 2021
Higgins 2020a
-
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020. Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2020b
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hoffmann 2021
Huang 2020
IntHout 2016
Jayk Bernal 2021
Johns Hopkins University of Medicine 2022
-
- Johns Hopkins University & Medicine Coronavirus Resource Center. Mortality analyses: How does mortality differ across countries? coronavirus.jhu.edu/data/mortality (accessed 10 February 2022).
Kacimi 2021
-
- Kacimi SE, Greca E, Haireche MA, ElHawary SA, Setti MO, Caruana R, et al. The place of fluvoxamine in the treatment of non-critically ill patients with COVID-19: a living systematic review and meta-analysis. medRxiv 2021. [DOI: 10.1101/2021.12.19.21268044] - DOI
Kam 1997
Kreuzberger 2021
Lauer 2020
Lee 2021
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Marshall 2020
Mikolajewska 2021
Murchu 2022
National Institutes of Health 2022
-
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. www.covid19treatmentguidelines.nih.gov/ (accessed 17 February 2022).
NCT04510194
-
- NCT04510194. COVID-OUT: early outpatient treatment for SARS-CoV-2 infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04510194 (first received 12 August 2020).
NCT04668950
-
- NCT04668950. Fluvoxamine for early treatment of Covid-19 (Stop Covid 2). clinicaltrials.gov/ct2/show/NCT04668950 (first received 16 December 2020).
NCT04718480
-
- NCT04718480. Fluvoxamine administration in moderate SARS-CoV-2 (COVID-19) infected patients. clinicaltrials.gov/ct2/show/NCT04718480 (first received 22 January 2021).
NCT04885530
-
- NCT04885530. ACTIV-6: COVID-19 outpatient randomized trial to evaluate efficacy of repurposed medications. clinicaltrials.gov/ct2/show/NCT04885530 (first received on 13 May 2021).
NCT05087381
-
- NCT05087381. Randomized-controlled trial of the effectiveness of COVID-19 early treatment in community. clinicaltrials.gov/ct2/show/record/NCT05087381 (first received 21 October 2021).
Oran 2020
Page 2021
Pan 2020
Piechotta 2022
-
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] - DOI - PMC - PubMed
Planas 2022
Popp 2021a
Popp 2021b
Rafiee 2016
RECOVERY 2021
RevMan Web 2022 [Computer program]
-
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available from revman.cochrane.org.
Rosen 2019
-
- Rosen DA, Seki SM, Fernández-Castañeda A, Beiter RM, Eccles JD, Woodfolk JA, et al. Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Science Translational Medicine 2019;11(478):5266. [DOI: 10.1126/scitranslmed.aau5266] - DOI - PMC - PubMed
Safa 2020
-
- Safa M, Hashemian MR, Abedi M, Malek MM. Effect of fluvoxamine medicine on cytokine level of COVID-19 patients, hospitalized in ICU ward [تأثیر داروی فلووکسامین بر سطح سیتوکین بیماران مبتال به9-COVID بستری در بخش ICU]. Journal of Medical Council of Iran 2020;30(3):135-8. [URL: jmciri.ir/browse.php?a_code=A-10-1-2643&sid=1&slc_lang=en]
Santesso 2020
Schünemann 2020
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Singh 2021
Skoetz 2020
-
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and Evidence Profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI: 10.1016/j.jclinepi.2019.10.015] - DOI - PubMed
Sterne 2019
Stroehlein 2021
-
- Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf MI, Benstoem C, et al. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD015043. [DOI: 10.1002/14651858.CD015043] - DOI - PMC - PubMed
Struyf 2020
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] - DOI - PMC - PubMed
Sukhatme 2021
Takenaka 2022
-
- Takenaka Y, Tanaka R, Kitabatake K, Kuramochi K, Aoki S, Tsukimoto M. Profiling differential effects of 5 selective serotonin reuptake inhibitors on TLRs-dependent and -independent IL-6 production in immune cells identifies fluoxetine as preferred anti-inflammatory drug candidate. Frontiers in Pharmacology 2022;13:874375. [DOI: 10.3389/fphar.2022.874375] - DOI - PMC - PubMed
TCTR20210615002
-
- TCTR20210615002. Effect of combined fluvoxamine with favipiravir versus favipiravir monotherapy in prevention of clinical deterioration among mild to moderate COVID-19 patients monitoring by telemedicine in virtual clinic: open-label randomized controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210615002 (first received 14 June 2021). [CENTRAL: CN-02281273]
van Harten 1995
Wagner 2021
Weinreich 2021
Wen 2022
WHO 2003
-
- World Health Organization. Cumulative number of reported probable cases of SARS; 2003. www.who.int/csr/sars/country/2003_07_11/en (accessed 16 December 2021).
WHO 2019a
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV): 2019. www.who.int/emergencies/mers-cov/en (accessed 6 December 2021).
WHO 2020a
-
- World Health Organization. Report of the WHO-China JointMission on coronavirus disease 2019 (COVID-19). www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-onc... (accessed 6 December 2021).
WHO 2020b
-
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020 (accessed 6 December 2021).
WHO 2020c
-
- World Health Organization. DraR landscape of COVID-19 candidate vaccines. www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed 6 December 2021).
WHO 2020e
-
- World Health Organization. COVID-19 Therapeutic Trial Synopsis. www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis (accessed 14 February 2022).
WHO 2021a
-
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 25 February 2022).
WHO 2021b
-
- World Health Organization. SARS-CoV-2 Variants. www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed 6 December 2021).
WHO 2022
-
- World Health Organization. Tracking SARS-CoV-2 variants. www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed 18 February 2022). - PubMed
WHO2022a
-
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. covid19.who.int/ (accessed 25 February 2022).
Williamson 2020
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

