Switchable Cycloadditions of Mesoionic Dipoles: Refreshing up a Regioselective Approach to Two Distinctive Heterocycles
- PMID: 36103345
- PMCID: PMC9552231
- DOI: 10.1021/acs.joc.2c01444
Switchable Cycloadditions of Mesoionic Dipoles: Refreshing up a Regioselective Approach to Two Distinctive Heterocycles
Abstract
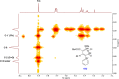
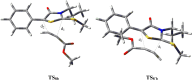
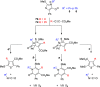
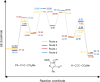
Mesoionic rings are among the most versatile 1,3-dipoles, as witnessed recently by their incorporation into bio-orthogonal strategies, and capable of affording unconventional heterocycles beyond the expected scope of Huisgen cycloadditions. Herein, we revisit in detail the reactivity of thiazol-3-ium-4-olates with alkynes, leading to thiophene and/or pyrid-2-one derivatives. A structural variation at the parent mesoionic dipole alters sufficiently the steric outcome, thereby favoring the regioselective formation of a single transient cycloadduct, which undergoes chemoselective fragmentation to either five- or six-membered heterocycles. The synthetic protocol benefits largely from microwave (MW) activation, which enhances reaction rates. The mechanism has been interrogated with the aid of density functional theory (DFT) calculations, which sheds light into the origin of the regioselectivity and points to a predictive formulation of reactivity involving competing pathways of mesoionic cycloadditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

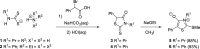






References
-
- Li J. J.Heterocyclic Chemistry in Drug Discovery; Wiley: New York, 2013.
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. - DOI - PubMed
- Cabrele C.; Reiser O. The modern face of synthetic heterocyclic chemistry. J. Org. Chem. 2016, 81, 10109–10125. 10.1021/acs.joc.6b02034. - DOI - PubMed
- Rojas-Buzo S.; García-García P.; Corma A. Remarkable acceleration of benzimidazole synthesis and cyanosilylation reactions in a supramolecular solid catalyst. ChemCatChem 2017, 9, 997–1004. 10.1002/cctc.201601407. - DOI
- Clarke A. K.; Unsworth W. P. A happy medium: the synthesis of medicinally important medium-sized rings via ring expansion. Chem. Sci. 2020, 11, 2876–2881. 10.1039/d0sc00568a. - DOI - PMC - PubMed
- Afanasenko A.; Barta K. Pharmaceutically relevant (hetero)cyclic compounds and natural products from lignin-derived monomers: present and perspectives. iScience 2021, 24, 102211.10.1016/j.isci.2021.102211. - DOI - PMC - PubMed
-
-
For recent and general overviews on mesoionic cycloadditions, spanning the past two decades:
- Gribble G. W.Mesoionic ring systems. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products Chemistry of Heterocyclic Compounds; Padwa A., Pearson W. H., Eds.; Wiley: New York, 2002; Vol. 59, pp 681–753.
- Ávalos M.; Babiano R.; Cintas P.; Jiménez J. L.; Palacios J. C. Exploiting Synthetic Chemistry with Mesoionic Rings: Improvements Achieved with Thioisomünchnones. Acc. Chem. Res. 2005, 38, 460–468. 10.1021/ar040212r. - DOI - PubMed
- Lopchuk J. M.Mesoionics. In Metalation of Azoles and Related Five-Membered Ring Heterocycles Topics in Heterocyclic Chemistry; Gribble G. W., Ed.; Springer: Berlin, 2012; Vol. 29, pp. 381–413.
- Moderhack D. Mesoionic Tetrazoles - Progress Since 1980. Heterocycles 2016, 92, 185–233. 10.3987/rev-15-833. - DOI
- García de la Concepción J.; Martínez R. F.; Cintas P.; Jiménez J. L.. Cycloadditions with mesoionic dipoles: strategy and control. In Targets in Heterocyclic Systems; Attanasi O., Merino P., Spinelli D., Eds.; Società Chimica Italiana: Rome, 2017; Ch. 11, Vol. 21, pp 228–253.
- Ramsden C. A.Heterocyclic Mesomeric Betaines and Mesoionic Compounds. Advances in Heterocyclic Chemistry; Academic Press: New York, 2022; Vol. 137, Ch. 3 and 4 for advances between 1980 and 2020.
-
-
- For a classical review describing the classification and conjugation of mesomeric betaines:Ollis W. D.; Stanforth S. P.; Ramsden C. A. Heterocyclic mesomeric betaines. Tetrahedron 1985, 41, 2239–2329. 10.1016/s0040-4020(01)96625-6. - DOI
- On the intersection of conjugation and cycloaddition in mesomeric betaines: Schmidt A.Heterocyclic mesomeric betaines and analogs in natural product chemistry. Betainic alkaloids and nucleobases. In Advances in Heterocyclic Chemistry; Katritzky A., Ed.; Academic Press: San Diego, 2003; Vol. 85, pp 67–171. For other studies aimed at addressing delocalization and reactivity:
- Browne D. L.; Harrity J. P. A. Recent developments in the chemistry of sydnones. Tetrahedron 2010, 66, 553–568. 10.1016/j.tet.2009.10.085. - DOI
- Wiechmann S.; Freese T.; Drafz M. H. H.; Hübner E. G.; Namyslo J. C.; Nieger M.; Schmidt A. Sydnone anions and abnormal N-heterocyclic carbenes of O-ethylsydnones. Characterizations, calculations and catalyses. Chem. Commun. 2014, 50, 11822–11824. 10.1039/c4cc05461j. - DOI - PubMed
- Oziminski W. P.; Ramsden C. A. A DFT and ab initio study of conjugated and semi-conjugated mesoionic rings and their covalent isomers. Tetrahedron 2015, 71, 7191–7198. 10.1016/j.tet.2015.01.045. - DOI
- Champagne P. A.; Houk K. N. Influence of Endo- and Exocyclic Heteroatoms on Stabilities and 1,3-Dipolar Cycloaddition Reactivities of Mesoionic Azomethine Ylides and Imines. J. Org. Chem. 2017, 82, 10980–10988. 10.1021/acs.joc.7b01928. - DOI - PMC - PubMed
-
- Narayanam M. K.; Liang Y.; Houk K. N.; Murphy J. M. Discovery of new mutually orthogonal bioorthogonal cycloaddition pairs through computational screening. Chem. Sci. 2016, 7, 1257–1261. 10.1039/c5sc03259h. - DOI - PMC - PubMed
- García de la Concepción J.; Avalos M.; Cintas P.; Jiménez J. L. Computational screening of new orthogonal metal-free dipolar cycloadditions of mesomeric betaines. Chem.—Eur. J. 2018, 24, 7507–7512. 10.1002/chem.201800869. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

