Loss of Sirtuin 1 (SIRT1) potentiates endothelial dysfunction via impaired glycolysis during infectious challenge
- PMID: 36103428
- PMCID: PMC9473483
- DOI: 10.1002/ctm2.1054
Loss of Sirtuin 1 (SIRT1) potentiates endothelial dysfunction via impaired glycolysis during infectious challenge
Conflict of interest statement
None declared.
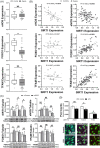
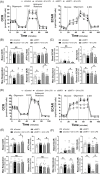
Figures


Similar articles
-
Endothelial SIRT1 as a Target for the Prevention of Arterial Aging: Promises and Challenges.J Cardiovasc Pharmacol. 2021 Dec 1;78(Suppl 6):S63-S77. doi: 10.1097/FJC.0000000000001154. J Cardiovasc Pharmacol. 2021. PMID: 34840264 Review.
-
Sirtuin 1 ablation in endothelial cells is associated with impaired angiogenesis and diastolic dysfunction.Am J Physiol Heart Circ Physiol. 2014 Dec 15;307(12):H1691-704. doi: 10.1152/ajpheart.00281.2014. Epub 2014 Sep 19. Am J Physiol Heart Circ Physiol. 2014. PMID: 25239805 Free PMC article.
-
Childhood psychosocial stress is linked with impaired vascular endothelial function, lower SIRT1, and oxidative stress in young adulthood.Am J Physiol Heart Circ Physiol. 2021 Sep 1;321(3):H532-H541. doi: 10.1152/ajpheart.00123.2021. Epub 2021 Jul 30. Am J Physiol Heart Circ Physiol. 2021. PMID: 34328346 Free PMC article.
-
Knockdown of circ0082374 inhibits cell viability, migration, invasion and glycolysis in glioma cells by miR-326/SIRT1.Brain Res. 2020 Dec 1;1748:147108. doi: 10.1016/j.brainres.2020.147108. Epub 2020 Sep 4. Brain Res. 2020. PMID: 32896523
-
Sirtuin 1 and Alzheimer's disease: An up-to-date review.Neuropeptides. 2018 Oct;71:54-60. doi: 10.1016/j.npep.2018.07.001. Epub 2018 Jul 9. Neuropeptides. 2018. PMID: 30007474 Review.
Cited by
-
Impact of FASN-enriched EVs on endothelial cell function in obstructive sleep apnea hypopnea syndrome.J Pharm Anal. 2025 May;15(5):101251. doi: 10.1016/j.jpha.2025.101251. Epub 2025 Mar 1. J Pharm Anal. 2025. PMID: 40521370 Free PMC article.
-
Research progress in the regulatory mechanism of silent information regulator 1 in sepsis (Review).Mol Med Rep. 2025 Aug;32(2):208. doi: 10.3892/mmr.2025.13573. Epub 2025 May 26. Mol Med Rep. 2025. PMID: 40417877 Free PMC article. Review.
-
Protein-mediated interactions in the dynamic regulation of acute inflammation.Biocell. 2023;47(6):1191-1198. doi: 10.32604/biocell.2023.027838. Epub 2023 May 19. Biocell. 2023. PMID: 37261220 Free PMC article.
References
-
- Shankar‐Hari M, Harrison DA, Rubenfeld GD, Rowan K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis‐2 and sepsis‐3 populations using a national critical care database. Br J Anaesth. 2017;119:626‐636. - PubMed
-
- Wang X, Zhang Q, Yan Y, Yang Y, Shang X, Li Y. Clinical Significance of pro‐inflammatory cytokines and their correlation with disease severity and blood coagulation in septic patients with bacterial co‐infection. Shock. 2021;56:396‐402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
