Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models
- PMID: 36103514
- PMCID: PMC9926941
- DOI: 10.1126/scitranslmed.abq1945
Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models
Abstract
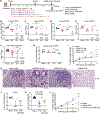
Emergence of SARS-CoV-2 variants of concern (VOCs), including the highly transmissible Omicron and Delta strains, has posed constant challenges to the current COVID-19 vaccines that principally target the viral spike protein (S). Here, we report a nucleoside-modified messenger RNA (mRNA) vaccine that expresses the more conserved viral nucleoprotein (mRNA-N) and show that mRNA-N vaccination alone can induce modest control of SARS-CoV-2. Critically, combining mRNA-N with the clinically proven S-expressing mRNA vaccine (mRNA-S+N) induced robust protection against both Delta and Omicron variants. In the hamster models of SARS-CoV-2 VOC challenge, we demonstrated that, compared to mRNA-S alone, combination mRNA-S+N vaccination not only induced more robust control of the Delta and Omicron variants in the lungs but also provided enhanced protection in the upper respiratory tract. In vivo CD8+ T cell depletion suggested a potential role for CD8+ T cells in protection conferred by mRNA-S+N vaccination. Antigen-specific immune analyses indicated that N-specific immunity, as well as augmented S-specific immunity, was associated with enhanced protection elicited by the combination mRNA vaccination. Our findings suggest that combined mRNA-S+N vaccination is an effective approach for promoting broad protection against SARS-CoV-2 variants.
Figures




References
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team, A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382, 727–733 (2020). - PMC - PubMed
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL, A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). - PMC - PubMed
-
- Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE, Briner C, Kwatra G, Ahmed K, Aley P, Bhikha S, Bhiman JN, Bhorat AE, du Plessis J, Esmail A, Groenewald M, Horne E, Hwa SH, Jose A, Lambe T, Laubscher M, Malahleha M, Masenya M, Masilela M, McKenzie S, Molapo K, Moultrie A, Oelofse S, Patel F, Pillay S, Rhead S, Rodel H, Rossouw L, Taoushanis C, Tegally H, Thombrayil A, van Eck S, Wibmer CK, Durham NM, Kelly EJ, Villafana TL, Gilbert S, Pollard AJ, de Oliveira T, Moore PL, Sigal A, Izu A; NGS-SA Group; Wits-VIDA COVID Group, Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 384, 1885–1898 (2021). - PMC - PubMed
-
- Moore JP, Offit PA, SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA 325, 821–822 (2021). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 AI127744/AI/NIAID NIH HHS/United States
- R01 AI132674/AI/NIAID NIH HHS/United States
- R01 AI134907/AI/NIAID NIH HHS/United States
- R21 AI147903/AI/NIAID NIH HHS/United States
- R01 AI147394/AI/NIAID NIH HHS/United States
- HHSN272201600013C/AI/NIAID NIH HHS/United States
- P01 AI158571/AI/NIAID NIH HHS/United States
- R24 AI120942/AI/NIAID NIH HHS/United States
- R43 AI145617/AI/NIAID NIH HHS/United States
- U01 AI056536/AI/NIAID NIH HHS/United States
- R01 AI157852/AI/NIAID NIH HHS/United States
- R01 AI112844/AI/NIAID NIH HHS/United States
- R21 AI140569/AI/NIAID NIH HHS/United States
- R01 AI154598/AI/NIAID NIH HHS/United States
- UL1 TR001439/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

