Ion slippage through Li+-centered G-quadruplex
- PMID: 36103528
- PMCID: PMC9473610
- DOI: 10.1126/sciadv.abp8751
Ion slippage through Li+-centered G-quadruplex
Abstract
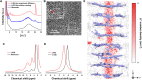
Single-ion conductors have garnered attention in energy storage systems as a promising alternative to currently widespread electrolytes that allow migration of cations and anions. However, ion transport phenomena of most single-ion conductors are affected by strong ion (e.g., Li+)-ion (immobilized anionic domains) interactions and tortuous paths, which pose an obstacle to achieving performance breakthroughs. Here, we present a Li+-centered G-quadruplex (LiGQ) as a class of single-ion conductor based on directional Li+ slippage at the microscopic level. A guanine derivative with liquid crystalline moieties is self-assembled to form a hexagonal ordered columnar structure in the LiGQ, thereby yielding one-dimensional central channels that provide weak ion-dipole interaction and straightforward ionic pathways. The LiGQ exhibits weak Li+ binding energy and low activation energy for ion conduction, verifying its viability as a new electrolyte design.
Figures



References
-
- Eperon G. E., Hörantner M. T., Snaith H. J., Metal halide perovskite tandem and multiple-junction photovoltaics. Nat. Rev. Chem. 1, 0095 (2017).
-
- Yang C., Suo Z., Hydrogel ionotronics. Nat. Rev. Mater. 3, 125–142 (2018).
-
- Kim H. J., Chen B., Suo Z., Hayward R. C., Ionoelastomer junctions between polymer networks of fixed anions and cations. Science 367, 773–776 (2020). - PubMed
-
- Zhang W., Seo D.-H., Chen T., Wu L., Topsakal M., Zhu Y., Lu D., Ceder G., Wang F., Kinetic pathways of ionic transport in fast-charging lithium titanate. Science 367, 1030–1034 (2020). - PubMed
-
- Rustomji C. S., Yang Y., Kim T. K., Mac J., Kim Y. J., Caldwell E., Chung H., Meng Y. S., Liquefied gas electrolytes for electrochemical energy storage devices. Science 356, eaal4263 (2017). - PubMed
LinkOut - more resources
Full Text Sources

