Biology of lung macrophages in health and disease
- PMID: 36103853
- PMCID: PMC9533769
- DOI: 10.1016/j.immuni.2022.08.010
Biology of lung macrophages in health and disease
Abstract
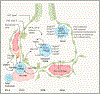
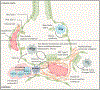
Tissue-resident alveolar and interstitial macrophages and recruited macrophages are critical players in innate immunity and maintenance of lung homeostasis. Until recently, assessing the differential functional contributions of tissue-resident versus recruited macrophages has been challenging because they share overlapping cell surface markers, making it difficult to separate them using conventional methods. This review describes how scRNA-seq and spatial transcriptomics can separate these subpopulations and help unravel the complexity of macrophage biology in homeostasis and disease. First, we provide a guide to identifying and distinguishing lung macrophages from other mononuclear phagocytes in humans and mice. Second, we outline emerging concepts related to the development and function of the various lung macrophages in the alveolar, perivascular, and interstitial niches. Finally, we describe how different tissue states profoundly alter their functions, including acute and chronic lung disease, cancer, and aging.
Keywords: alveolar macrophages; interstitial macrophages; lung disease; pulmonary; recruited macrophages.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Antonelli LRV, Gigliotti Rothfuchs A, Gonçalves R, Roffê E, Cheever AW, Bafica A, Salazar AM, Feng CG, and Sher A (2010). Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest 120, 1674–1682. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

