GABBR1 monoallelic de novo variants linked to neurodevelopmental delay and epilepsy
- PMID: 36103875
- PMCID: PMC9606381
- DOI: 10.1016/j.ajhg.2022.08.010
GABBR1 monoallelic de novo variants linked to neurodevelopmental delay and epilepsy
Abstract
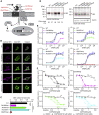
GABAB receptors are obligatory heterodimers responsible for prolonged neuronal inhibition in the central nervous system. The two receptor subunits are encoded by GABBR1 and GABBR2. Variants in GABBR2 have been associated with a Rett-like phenotype (MIM: 617903), epileptic encephalopathy (MIM: 617904), and milder forms of developmental delay with absence epilepsy. To date, however, no phenotypes associated with pathogenic variants of GABBR1 have been established. Through GeneMatcher, we have ascertained four individuals who each have a monoallelic GABBR1 de novo non-synonymous variant; these individuals exhibit motor and/or language delay, ranging from mild to severe, and in one case, epilepsy. Further phenotypic features include varying degrees of intellectual disability, learning difficulties, autism, ADHD, ODD, sleep disorders, and muscular hypotonia. We functionally characterized the four de novo GABBR1 variants, p.Glu368Asp, p.Ala397Val, p.Ala535Thr, and p.Gly673Asp, in transfected HEK293 cells. GABA fails to efficiently activate the variant receptors, most likely leading to an increase in the excitation/inhibition balance in the central nervous system. Variant p.Gly673Asp in transmembrane domain 3 (TMD3) renders the receptor completely inactive, consistent with failure of the receptor to reach the cell surface. p.Glu368Asp is located near the orthosteric binding site and reduces GABA potency and efficacy at the receptor. GABA exhibits normal potency but decreased efficacy at the p.Ala397Val and p.Ala535Thr variants. Functional characterization of GABBR1-related variants provides a rationale for understanding the severity of disease phenotypes and points to possible therapeutic strategies.
Keywords: GABBR1; gene; mendelian disease.
Copyright © 2022 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.E.A. is a cofounder and CEO of Medigenome, Swiss Institute of Genomic Medicine; he is also a member of the Scientific Advisory Board of the “Imagine Institute”, Paris. E.R. is also a cofounder and medical director of Medigenome, Swiss Institute of Genomic Medicine. M.L.C. is an intern in the federally recognized clinical training program for Genetic Medicine of Medigenome. L.N. was supported by the grant NV19-07-00136 from the Ministry of Health of the Czech Republi, and The National Center for Medical Genomics (LM2018132) for support with the WES analyses.
Figures
References
-
- Mariotti L., Losi G., Lia A., Melone M., Chiavegato A., Gómez-Gonzalo M., Sessolo M., Bovetti S., Forli A., Zonta M., et al. Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat. Commun. 2018;9:82. doi: 10.1038/s41467-017-02642-6. - DOI - PMC - PubMed
-
- Perea G., Gómez R., Mederos S., Covelo A., Ballesteros J.J., Schlosser L., Hernández-Vivanco A., Martín-Fernández M., Quintana R., Rayan A., et al. Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. Elife. 2016;5:e20362. doi: 10.7554/eLife.20362. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

