mTOR-regulated mitochondrial metabolism limits mycobacterium-induced cytotoxicity
- PMID: 36103894
- PMCID: PMC9596383
- DOI: 10.1016/j.cell.2022.08.018
mTOR-regulated mitochondrial metabolism limits mycobacterium-induced cytotoxicity
Abstract
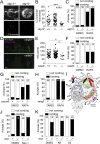
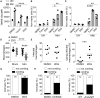
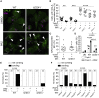
Necrosis of macrophages in the granuloma, the hallmark immunological structure of tuberculosis, is a major pathogenic event that increases host susceptibility. Through a zebrafish forward genetic screen, we identified the mTOR kinase, a master regulator of metabolism, as an early host resistance factor in tuberculosis. We found that mTOR complex 1 protects macrophages from mycobacterium-induced death by enabling infection-induced increases in mitochondrial energy metabolism fueled by glycolysis. These metabolic adaptations are required to prevent mitochondrial damage and death caused by the secreted mycobacterial virulence determinant ESAT-6. Thus, the host can effectively counter this early critical mycobacterial virulence mechanism simply by regulating energy metabolism, thereby allowing pathogen-specific immune mechanisms time to develop. Our findings may explain why Mycobacterium tuberculosis, albeit humanity's most lethal pathogen, is successful in only a minority of infected individuals.
Keywords: ESAT-6 mitotoxicity; Mycobacterium marinum; Mycobacterium tuberculosis; granuloma necrosis; mTOR; macrophage death; mitochondrial metabolism; oxidative phosphorylation; tuberculosis; zebrafish TB model.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.R. and E.L.P. are advisory board members for Cell. E.L.P. is a scientific advisory board member of ImmunoMet and a founder of Rheos Medicines. For the purpose of open access, the authors have applied for a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. This work is licensed under a Creative Commons Attribution 4.0 International License.
Figures









References
-
- Augenstreich J., Arbues A., Simeone R., Haanappel E., Wegener A., Sayes F., Le Chevalier F., Chalut C., Malaga W., Guilhot C., et al. ESX-1 and phthiocerol dimycocerosates of Mycobacterium tuberculosis act in concert to cause phagosomal rupture and host cell apoptosis. Cell. Microbiol. 2017;19:e12726. doi: 10.1111/cmi.12726. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

