Mesenchymal stem cells derived from patients with premature aging syndromes display hallmarks of physiological aging
- PMID: 36104080
- PMCID: PMC9475049
- DOI: 10.26508/lsa.202201501
Mesenchymal stem cells derived from patients with premature aging syndromes display hallmarks of physiological aging
Abstract
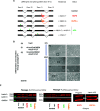
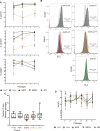
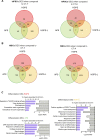
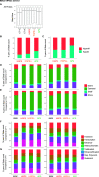
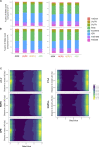
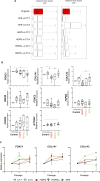
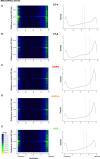
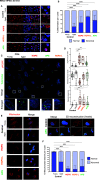
Progeroid syndromes are rare genetic diseases with most of autosomal dominant transmission, the prevalence of which is less than 1/10,000,000. These syndromes caused by mutations in the <i>LMNA</i> gene encoding A-type lamins belong to a group of disorders called laminopathies. Lamins are implicated in the architecture and function of the nucleus and chromatin. Patients affected with progeroid laminopathies display accelerated aging of mesenchymal stem cells (MSCs)-derived tissues associated with nuclear morphological abnormalities. To identify pathways altered in progeroid patients' MSCs, we used induced pluripotent stem cells (hiPSCs) from patients affected with classical Hutchinson-Gilford progeria syndrome (HGPS, c.1824C&gt;T-p.G608G), HGPS-like syndrome (HGPS-L; c.1868C&gt;G-p.T623S) associated with farnesylated prelamin A accumulation, or atypical progeroid syndromes (APS; homozygous c.1583C&gt; T-p.T528M; heterozygous c.1762T&gt;C-p.C588R; compound heterozygous c.1583C&gt;T and c.1619T&gt;C-p.T528M and p.M540T) without progerin accumulation. By comparative analysis of the transcriptome and methylome of hiPSC-derived MSCs, we found that patient's MSCs display specific DNA methylation patterns and modulated transcription at early stages of differentiation. We further explored selected biological processes deregulated in the presence of <i>LMNA</i> variants and confirmed alterations of age-related pathways during MSC differentiation. In particular, we report the presence of an altered mitochondrial pattern; an increased response to double-strand DNA damage; and telomere erosion in HGPS, HGPS-L, and APS MSCs, suggesting converging pathways, independent of progerin accumulation, but a distinct DNA methylation profile in HGPS and HGPS-L compared with APS cells.
© 2022 Trani et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Identification of mitochondrial dysfunction in Hutchinson-Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture.J Proteomics. 2013 Oct 8;91:466-77. doi: 10.1016/j.jprot.2013.08.008. Epub 2013 Aug 20. J Proteomics. 2013. PMID: 23969228
-
Differential stem cell aging kinetics in Hutchinson-Gilford progeria syndrome and Werner syndrome.Protein Cell. 2018 Apr;9(4):333-350. doi: 10.1007/s13238-018-0517-8. Epub 2018 Feb 23. Protein Cell. 2018. PMID: 29476423 Free PMC article.
-
Truncated prelamin A expression in HGPS-like patients: a transcriptional study.Eur J Hum Genet. 2015 Aug;23(8):1051-61. doi: 10.1038/ejhg.2014.239. Epub 2015 Feb 4. Eur J Hum Genet. 2015. PMID: 25649378 Free PMC article.
-
Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations.Ageing Res Rev. 2017 Jan;33:18-29. doi: 10.1016/j.arr.2016.06.007. Epub 2016 Jun 29. Ageing Res Rev. 2017. PMID: 27374873 Free PMC article. Review.
-
Hutchinson-Gilford progeria syndrome: unraveling the genetic basis, symptoms, and advancements in therapeutic approaches.Ther Adv Rare Dis. 2024 Dec 16;5:26330040241305144. doi: 10.1177/26330040241305144. eCollection 2024 Jan-Dec. Ther Adv Rare Dis. 2024. PMID: 39691184 Free PMC article. Review.
Cited by
-
The farnesyl transferase inhibitor (FTI) lonafarnib improves nuclear morphology in ZMPSTE24-deficient fibroblasts from patients with the progeroid disorder MAD-B.Nucleus. 2023 Dec;14(1):2288476. doi: 10.1080/19491034.2023.2288476. Epub 2023 Dec 5. Nucleus. 2023. PMID: 38050983 Free PMC article.
-
When do the pathological signs become evident? Study of human mesenchymal stem cells in MDPL syndrome.Aging (Albany NY). 2024 Nov 26;16(22):13505-13525. doi: 10.18632/aging.206159. Epub 2024 Nov 26. Aging (Albany NY). 2024. PMID: 39611849 Free PMC article.
-
Validation of metaxin-2 deficient C. elegans as a model for MandibuloAcral Dysplasia associated to mtx-2 (MADaM) syndrome.Commun Biol. 2024 Oct 26;7(1):1398. doi: 10.1038/s42003-024-06967-z. Commun Biol. 2024. PMID: 39462037 Free PMC article.
-
Cellular microenvironment: a key for tuning mesenchymal stem cell senescence.Front Cell Dev Biol. 2023 Dec 4;11:1323678. doi: 10.3389/fcell.2023.1323678. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38111850 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
