Tumor-associated macrophage-specific CD155 contributes to M2-phenotype transition, immunosuppression, and tumor progression in colorectal cancer
- PMID: 36104099
- PMCID: PMC9476138
- DOI: 10.1136/jitc-2021-004219
Tumor-associated macrophage-specific CD155 contributes to M2-phenotype transition, immunosuppression, and tumor progression in colorectal cancer
Abstract
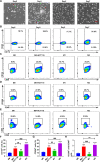
Background: Onco-immunogenic molecule CD155 is overexpressed in various tumor microenvironments (TME) including in colorectal cancer (CRC). Tumor-associated macrophages (TAMs) are the most abundant immune cells in CRC TME and play a vital role in CRC progression and metastasis. Most studies have focused on investigating the role of CRC cell-specific CD155 on CRC progression, while the contribution of TAMs-specific CD155 is still unknown. Here, we sought to investigate the expression pattern of CD155 in CRC TAMs and its role in tumor immunity and progression.
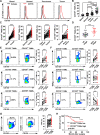
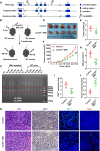
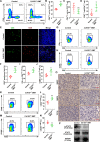
Methods: CD155 expression patterns in CRC TAMs and macrophages in paratumor or adjacent normal tissue were analyzed in 50 patients with CRC using flow cytometry and in 141 patients with CRC using immunohistochemistry. The correlation of CD155 expression level in TAMs with M1 and M2 phenotypic transition was analyzed. The role of macrophage-specific CD155 in CRC progression and tumor immune response was investigated in vitro and in vivo. We further analyzed the effect of CRC cells on the regulation of CD155 expression in macrophages.
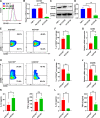
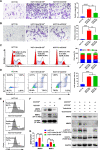
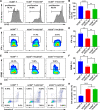
Results: CRC TAMs from clinical samples showed robustly higher expression of CD155 than macrophages from paratumor and adjacent normal tissues. The CD155 expression level was higher in TAMs of CRC at III/IV stages compared with the I/II stages and was negatively associated with the survival of patients with CRC. CD155+ TAMs showed an M2 phenotype and higher expression of interleukin (IL)-10 and transforming growth factor (TGF)-β. CD155+ macrophages promoted CRC cell migration, invasion, and tumor growth supporting the findings from the clinical tissue analysis. This effect was mainly regulated by TGF-β-induced STAT3 activation-mediated release of matrix metalloproteinases (MMP)2 and MMP9 in CRC cells. CD155-⁄- bone marrow transplantation in wild-type mice, as well as CD155- macrophages treatment, promoted the antitumor immune response in the mice ectopic CRC model. Additionally, CRC cells released IL-4 to trigger CD155 expression in macrophages indicating the regulatory role of CRC cells in the development of CD155+ TAMs.
Conclusions: These findings indicated that CD155+ TAMs are responsible for the M2-phenotype transition, immunosuppression, and tumor progression in CRC. The specific localization of CD155+ TAMs in CRC tissue could turn into a potential therapeutic target for CRC treatment.
Keywords: immunity; tumor escape; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
