Synthetic Mucin Gels with Self-Healing Properties Augment Lubricity and Inhibit HIV-1 and HSV-2 Transmission
- PMID: 36104216
- PMCID: PMC9661867
- DOI: 10.1002/advs.202203898
Synthetic Mucin Gels with Self-Healing Properties Augment Lubricity and Inhibit HIV-1 and HSV-2 Transmission
Abstract
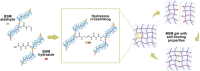
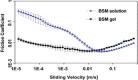
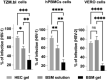
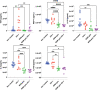
Mucus is a self-healing gel that lubricates the moist epithelium and provides protection against viruses by binding to viruses smaller than the gel's mesh size and removing them from the mucosal surface by active mucus turnover. As the primary nonaqueous components of mucus (≈0.2%-5%, wt/v), mucins are critical to this function because the dense arrangement of mucin glycans allows multivalence of binding. Following nature's example, bovine submaxillary mucins (BSMs) are assembled into "mucus-like" gels (5%, wt/v) by dynamic covalent crosslinking reactions. The gels exhibit transient liquefaction under high shear strain and immediate self-healing behavior. This study shows that these material properties are essential to provide lubricity. The gels efficiently reduce human immunodeficiency virus type 1 (HIV-1) and genital herpes virus type 2 (HSV-2) infectivity for various types of cells. In contrast, simple mucin solutions, which lack the structural makeup, inhibit HIV-1 significantly less and do not inhibit HSV-2. Mechanistically, the prophylaxis of HIV-1 infection by BSM gels is found to be that the gels trap HIV-1 by binding to the envelope glycoprotein gp120 and suppress cytokine production during viral exposure. Therefore, the authors believe the gels are promising for further development as personal lubricants that can limit viral transmission.
Keywords: HIV-1; HSV-2; immune suppression; lubricant; mucin hydrogels; self-healing; strain-weakening.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mucus and Mucins: do they have a role in the inhibition of the human immunodeficiency virus?Virol J. 2017 Oct 6;14(1):192. doi: 10.1186/s12985-017-0855-9. Virol J. 2017. PMID: 28985745 Free PMC article. Review.
-
Mucus-Inspired Self-Healing Hydrogels: A Protective Barrier for Cells against Viral Infection.Adv Mater. 2024 Aug;36(32):e2401745. doi: 10.1002/adma.202401745. Epub 2024 Jun 10. Adv Mater. 2024. PMID: 38815174
-
Broadly neutralizing antibodies consistently trap HIV-1 in fresh cervicovaginal mucus from select individuals.Acta Biomater. 2023 Oct 1;169:387-397. doi: 10.1016/j.actbio.2023.07.031. Epub 2023 Jul 26. Acta Biomater. 2023. PMID: 37499728 Free PMC article.
-
Influenza A virus diffusion through mucus gel networks.Commun Biol. 2022 Mar 22;5(1):249. doi: 10.1038/s42003-022-03204-3. Commun Biol. 2022. PMID: 35318436 Free PMC article.
-
Mucins, Mucus, and Goblet Cells.Chest. 2018 Jul;154(1):169-176. doi: 10.1016/j.chest.2017.11.008. Epub 2017 Nov 21. Chest. 2018. PMID: 29170036 Review.
Cited by
-
NET-EN treatment leads to delayed HSV-2 infection, enhanced mucin and T cell functions in the female genital tract when compared to DMPA in a preclinical mouse model.Front Immunol. 2024 Nov 6;15:1427842. doi: 10.3389/fimmu.2024.1427842. eCollection 2024. Front Immunol. 2024. PMID: 39569191 Free PMC article.
-
Mucosomes as next-generation drug carriers for treating mucus-resident bacterial infections and biofilms.Sci Rep. 2025 Jul 25;15(1):27071. doi: 10.1038/s41598-025-10496-y. Sci Rep. 2025. PMID: 40715188 Free PMC article.
-
Synthetic mucus biomaterials for antimicrobial peptide delivery.J Biomed Mater Res A. 2023 Oct;111(10):1616-1626. doi: 10.1002/jbm.a.37559. Epub 2023 May 18. J Biomed Mater Res A. 2023. PMID: 37199137 Free PMC article.
-
Preserving the Immune-Privileged Niche of the Nucleus Pulposus: Safeguarding Intervertebral Discs from Degeneration after Discectomy with Synthetic Mucin Hydrogel Injection.Adv Sci (Weinh). 2024 Nov;11(43):e2404496. doi: 10.1002/advs.202404496. Epub 2024 Aug 29. Adv Sci (Weinh). 2024. PMID: 39207014 Free PMC article.
-
Portable Quartz Crystal Resonator Sensor for Characterising the Gelation Kinetics and Viscoelastic Properties of Hydrogels.Gels. 2022 Nov 7;8(11):718. doi: 10.3390/gels8110718. Gels. 2022. PMID: 36354626 Free PMC article.
References
-
- McGuckin M. A., Lindén S. K., Sutton P., Florin T. H., Nat. Rev. Microbiol. 2011, 9, 265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
