Application of 3D Printing Technology to Produce Hippocampal Customized Guide Cannulas
- PMID: 36104275
- PMCID: PMC9522464
- DOI: 10.1523/ENEURO.0099-22.2022
Application of 3D Printing Technology to Produce Hippocampal Customized Guide Cannulas
Abstract
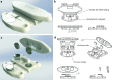
Implantation of guide cannulas is a widely used technique to access specific brain areas. Although commercially available, the need to personalize these implants and the high cost prompted us to design open-source customized devices taking advantage of 3D printing technology. Our cannulas consisted in a 3D-printed head mount designed according to the Paxinos coordinates to reach the CA1 area of the hippocampus. To cut guide cannulas to the proper length, we designed and realized an original 3D-printed linear motion apparatus. Polylactic acid thermoplastic polymer was used as printing material. Homemade or commercial cannulas were implanted in 4- to 6-month-old wild-type mice and intrahippocampal injections of amyloid-β peptide at different concentrations were performed. In vivo behavioral studies of novel object recognition indicated that results obtained with homemade versus commercial devices were comparable. Methylene blue injections and Nissl staining confirmed the correct localization of cannulas in the CA1 area of mouse hippocampus. Our method allows a fast manufacturing of hippocampal cannulas preserving the required precision at very low cost. Furthermore, this system can be easily modified to produce cannulas to target other brain areas. In conclusion, 3D printing might be used as a useful and versatile technology to realize open-source customized devices in neuroscience laboratories.
Keywords: 3D printing; behavioral studies; brain cannulas; hippocampus; neuroscience method; open source apparatus.
Copyright © 2022 Tropea et al.
Figures





Similar articles
-
Three-Dimensional Printing of a Transconjunctival Vitrectomy Trocar-Cannula System.Ophthalmologica. 2017;237(2):119-122. doi: 10.1159/000457807. Epub 2017 Mar 2. Ophthalmologica. 2017. PMID: 28249289
-
Two minimally invasive strategies to implant guide cannulas for multiple injections in deep brain areas.Methods. 2025 Jun;238:27-39. doi: 10.1016/j.ymeth.2025.03.005. Epub 2025 Mar 8. Methods. 2025. PMID: 40064209
-
Exploring the coating of 3D-printed insulating substrates with conductive composites: a simple, cheap and versatile strategy to prepare customized high-performance electrochemical sensors.Anal Methods. 2022 Sep 1;14(34):3345-3354. doi: 10.1039/d2ay00803c. Anal Methods. 2022. PMID: 35979860
-
3D Printing Technology in Customized Drug Delivery System: Current State of the Art, Prospective and the Challenges.Curr Pharm Des. 2018;24(42):5049-5061. doi: 10.2174/1381612825666190110153742. Curr Pharm Des. 2018. PMID: 30636582 Review.
-
Role of Polymers in 3D Printing Technology for Drug Delivery - An Overview.Curr Pharm Des. 2018;24(42):4979-4990. doi: 10.2174/1381612825666181226160040. Curr Pharm Des. 2018. PMID: 30585543 Review.
Cited by
-
An Open-Source 3D-Printed Recording Stage with Customizable Chambers for Ex Vivo Experiments.eNeuro. 2024 Sep 13;11(9):ENEURO.0257-24.2024. doi: 10.1523/ENEURO.0257-24.2024. Print 2024 Sep. eNeuro. 2024. PMID: 39197950 Free PMC article.
-
3-Dimensional printing and bioprinting in neurological sciences: applications in surgery, imaging, tissue engineering, and pharmacology and therapeutics.J Mater Sci Mater Med. 2025 Apr 9;36(1):32. doi: 10.1007/s10856-025-06877-4. J Mater Sci Mater Med. 2025. PMID: 40205004 Free PMC article. Review.
-
Blockade of dopamine D3 receptors improves hippocampal synaptic function and rescues age-related cognitive phenotype.Aging Cell. 2024 Nov;23(11):e14291. doi: 10.1111/acel.14291. Epub 2024 Sep 5. Aging Cell. 2024. PMID: 39236310 Free PMC article.
References
-
- Dunham S, Mosadegh B, Romito EA, Zgaren M (2018) Application of 3D printing. In: 3D printing applications in cardiovascular medicine (Al'Aref SJ, Mosadegh B, Dunham S, Min JK, eds), pp 61–78. London: Academic.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
