A biomarker assay to risk-stratify patients with symptoms of respiratory tract infection
- PMID: 36104292
- PMCID: PMC9753477
- DOI: 10.1183/13993003.00459-2022
A biomarker assay to risk-stratify patients with symptoms of respiratory tract infection
Abstract
Background: Patients who present to an emergency department (ED) with respiratory symptoms are often conservatively triaged in favour of hospitalisation. We sought to determine if an inflammatory biomarker panel that identifies the host response better predicts hospitalisation in order to improve the precision of clinical decision making in the ED.
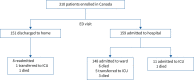
Methods: From April 2020 to March 2021, plasma samples of 641 patients with symptoms of respiratory illness were collected from EDs in an international multicentre study: Canada (n=310), Italy (n=131) and Brazil (n=200). Patients were followed prospectively for 28 days. Subgroup analysis was conducted on confirmed coronavirus disease 2019 (COVID-19) patients (n=245). An inflammatory profile was determined using a rapid, 50-min, biomarker panel (RALI-Dx (Rapid Acute Lung Injury Diagnostic)), which measures interleukin (IL)-6, IL-8, IL-10, soluble tumour necrosis factor receptor 1 (sTNFR1) and soluble triggering receptor expressed on myeloid cells 1 (sTREM1).
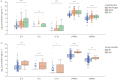
Results: RALI-Dx biomarkers were significantly elevated in patients who required hospitalisation across all three sites. A machine learning algorithm that was applied to predict hospitalisation using RALI-Dx biomarkers had a mean±sd area under the receiver operating characteristic curve of 76±6% (Canada), 84±4% (Italy) and 86±3% (Brazil). Model performance was 82±3% for COVID-19 patients and 87±7% for patients with a confirmed pneumonia diagnosis.
Conclusions: The rapid diagnostic biomarker panel accurately identified the need for inpatient care in patients presenting with respiratory symptoms, including COVID-19. The RALI-Dx test is broadly and easily applicable across many jurisdictions, and represents an important diagnostic adjunct to advance ED decision-making protocols.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: S. Keshavjee serves as Chief Medical Officer of Traferox Technologies and receives personal fees from Lung Bioengineering, outside the submitted work. A.T. Sage, M. Cypel, L. del Sorbo, B. Wang, J. Valero and S. Keshavjee are inventors of a patent licensed to SQI Diagnostics. The inventors fully adhere to a number of policies in place at the University Health Network that ensure academic integrity and management of potential conflicts of interest between authors and industry partners. For the purposes of regulatory application, SQI Diagnostics provided funding to support Brazil data collection and the work performed by D.R. Marinowic, F.O. Friedrich, C.R.R. Schmitz, L.S.M. dos Santos, F.M. Barbe-Tuana and M.H. Jones, but remained arms-length and blinded from the data analysis included in this study. All other authors report no conflicts of interest.
Figures

Comment in
-
A leap towards personalised therapy of acute lung injury.Eur Respir J. 2022 Dec 15;60(6):2201808. doi: 10.1183/13993003.01808-2022. Print 2022 Dec. Eur Respir J. 2022. PMID: 36522140 No abstract available.
References
-
- Canadian Institute for Health Information . Data Quality Documentation: National Ambulatory Care Reporting System – Current-Year Information 2018–2019. 2019. www.cihi.ca/sites/default/files/document/current-year-information-nacrs-... Date last accessed: 1 August 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
