Understanding the Surprising Oxidation Chemistry of Au-OH Complexes
- PMID: 36104296
- PMCID: PMC10091708
- DOI: 10.1002/cphc.202200475
Understanding the Surprising Oxidation Chemistry of Au-OH Complexes
Abstract
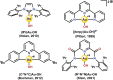
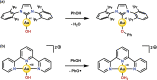
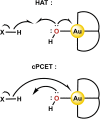
Au is known to be fairly redox inactive (in catalysis) and bind oxygen adducts only quite weakly. It is thus rather surprising that stable Au-OH complexes can be synthesized and used as oxidants for both one- and two-electron oxidations. A charged AuIII -OH complex has been shown to cleave C-H and O-H bonds homolytically, resulting in a one-electron reduction of the metal center. Contrasting this, a neutral AuIII -OH complex performs oxygen atom transfer to phosphines, resulting in a two-electron reduction of the hydroxide proton to form a AuIII -H rather than causing a change in oxidation state of the metal. We explore the details of these two examples and draw comparisons to the more conventional reactivity exhibited by AuI -OH. Although the current scope of known Au-OH oxidation chemistry is still in its infancy, the current literature exemplifies the unique properties of Au chemistry and shows promise for future findings in the field.
Keywords: gold hydroxides; mechanisms; oxidation; oxygen atom transfer; proton coupled electron transfer.
© 2022 The Authors. ChemPhysChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
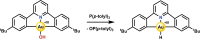
References
-
- Ikeda S., Nakajima T., Hirao K., Mol. Phys. 2003, 101, 105–110.
-
- Kayode G. O., Montemore M. M., J. Mater. Chem. A 2021, 9, 22325–22333.
-
- None
-
- Hayashi T., Tanaka K., Haruta M., J. Catal. 1998, 178, 566–575;
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

