Cytotoxic CD8+ T cells may be drivers of tissue destruction in Sjögren's syndrome
- PMID: 36104369
- PMCID: PMC9475031
- DOI: 10.1038/s41598-022-19397-w
Cytotoxic CD8+ T cells may be drivers of tissue destruction in Sjögren's syndrome
Abstract
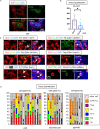
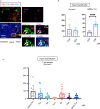
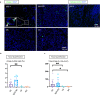
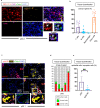
Sjögren's syndrome is a chronic autoimmune disorder whose pathogenesis is poorly understood and that lacks effective therapies. Detailed quantitative and spatial analyses of tissues affected by Sjögren's syndrome were undertaken, including the quantitation of the frequency of selected cell-cell interactions in the disease milieu. Quantitative analyses of CD4+ T cell subsets and of CD8+ T cells in the labial salivary glands from untreated patients with primary Sjögren's syndrome revealed that activated CD8+ cytotoxic T cells (CD8+CTLs) were the most prominent T cells in these infiltrates. An accumulation of apoptotic glandular epithelial cells, mainly ductal and acinar cells, was observed, consistent with the impaired salivary secretion often observed in patients with this disease. FasL expressing activated CD8+ T cells were seen to accumulate around Fas expressing apoptotic epithelial cells. Quantitative analyses of apoptotic cell types and of conjugates between cytotoxic T cells and epithelial cells undergoing apoptosis suggest that Sjögren's syndrome is primarily driven by CD8+CTL mediated execution of epithelial cells mainly represented by ductal and acinar cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

