SARS-CoV-2 can infect human embryos
- PMID: 36104397
- PMCID: PMC9472724
- DOI: 10.1038/s41598-022-18906-1
SARS-CoV-2 can infect human embryos
Abstract
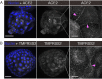
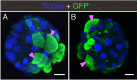
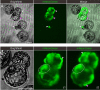
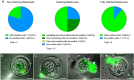
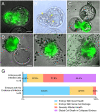
The spread of SARS-CoV-2 has led to a devastating pandemic, with infections resulting in a range of symptoms collectively known as COVID-19. The full repertoire of human tissues and organs susceptible to infection is an area of active investigation, and some studies have implicated the reproductive system. The effects of COVID-19 on human reproduction remain poorly understood, and particularly the impact on early embryogenesis and establishment of a pregnancy are not known. In this work, we explore the susceptibility of early human embryos to SARS-CoV-2 infection. By using RNA-seq and immunofluorescence, we note that ACE2 and TMPRSS2, two canonical cell entry factors for SARS-CoV-2, are co-expressed in cells of the trophectoderm in blastocyst-stage preimplantation embryos. For the purpose of viral entry studies, we used fluorescent reporter virions pseudotyped with Spike (S) glycoprotein from SARS-CoV-2, and we observe robust infection of trophectoderm cells. This permissiveness could be attenuated with blocking antibodies targeting S or ACE2. When exposing human blastocysts to the live, fully infectious SARS-CoV-2, we detected cases of infection that compromised embryo health. Therefore, we identify a new human target tissue for SARS-CoV-2 with potential medical implications for reproductive health during the COVID-19 pandemic and its aftermath.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

