The p-ERG spatial acuity in the biomedical pig under physiological conditions
- PMID: 36104429
- PMCID: PMC9474814
- DOI: 10.1038/s41598-022-19925-8
The p-ERG spatial acuity in the biomedical pig under physiological conditions
Abstract
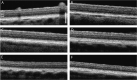
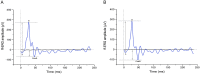
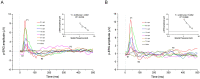
Pigs are becoming an important pre-clinical animal species for translational ophthalmology, due to similarities with humans in anatomical and physiological patterns. Different models of eye disorders have been proposed, and they are good candidates to assess biocompatibility/functionality of retinal prostheses. Electroretinography is a common tool allowing to gain information on retinal function, with several types of electroretinogram (ERG) been implemented including full field (ff-ERG), multifocal (mf-ERG) and pattern (p-ERG). p-ERG represents a valuable tool to monitor Retinal Ganglion Cells (RGCs) activity and can be used to calculate p-ERG spatial acuity. Unfortunately, scarce methodological data are available regarding recording/interpretation of p-ERG and retinal acuity in biomedical pigs yet enhancing knowledge regarding pig vision physiology will allow for more refined and responsible use of such species. Aim of this study was to record p-ERG in juvenile pigs to functionally assess visual acuity. Six female hybrid pigs underwent two p-ERG recording sessions at 16 and 19 weeks of age. Photopic ff-ERG were also recorded; optical coherence tomography (OCT) and histology were used to confirm retinal integrity. ff-ERG signals were repeatable within/across sessions. All p-ERG traces consistently displayed characterizing peaks, and the progressive decrease of amplitude in response to the increment of spatial frequency revealed the reliability of the method. Mean p-ERG spatial acuities were 5.7 ± 0.14 (16 weeks) and 6.2 ± 0.15 cpd (19 weeks). Overall, the p-ERG recordings described in the present work seem reliable and repeatable, and may represent an important tool when it comes to vision assessment in pigs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

