Ficus dubia latex extract prevent DMH-induced rat early colorectal carcinogenesis through the regulation of xenobiotic metabolism, inflammation, cell proliferation and apoptosis
- PMID: 36104433
- PMCID: PMC9474822
- DOI: 10.1038/s41598-022-19843-9
Ficus dubia latex extract prevent DMH-induced rat early colorectal carcinogenesis through the regulation of xenobiotic metabolism, inflammation, cell proliferation and apoptosis
Abstract
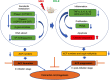
Ficus dubia latex is recognized as a remedy in Asian traditional medicine with various therapeutic effects. The present study aimed to determine the preventive action of Ficus dubia latex extract (FDLE) on 1,2-dimethylhydrazine (DMH)-induced rat colorectal carcinogenesis and its mechanisms. The experiment included an initiation model in which rats were orally administered with FDLE daily for 1 week before DMH injection until the end of the experiment, while only after DMH injection until the end in the post-initiation model. The results firstly indicated that FDLE treatment could reduce the level of methylazoxymethanol (MAM) in rat colonic lumen by inhibition of the activities of both phase I xenobiotic metabolizing enzymes in the liver and β-glucuronidase in the colon, leading to reduced DNA methylation in colonic mucosal cells, related to the number of ACF in the initiation stage. Besides, FDLE modulated the inflammation which could suppress the growth and induce apoptosis of aberrant colonic mucosal cells, leading to retardation of ACF multiplicity. Therefore, FDLE showed the ability to suppress the DMH-induced rat ACF formation and inflammation promoted growth of ACF. In conclusion, FDLE had the potential to prevent carcinogens-induced rat colorectal carcinogenesis in the initiation stage.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



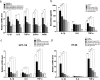

References
-
- Okada K, et al. Combination of the histone deacetylase inhibitor depsipeptide and 5-fluorouracil upregulates major histocompatibility complex class II and p21 genes and activates caspase-3/7 in human colon cancer HCT-116 cells. Oncol. Rep. 2016;36:1875–1885. doi: 10.3892/or.2016.5008. - DOI - PMC - PubMed
-
- Manju V, Nalini N. Effect of ginger on lipid peroxidation and antioxidant status in 1, 2-dimethyl hydrazine induced experimental colon carcinogenesis. J. Biochem. Technol. 2010;2:161–167.
-
- Khanaree C, Pintha K, Tantipaiboonwong P, Suttajit M, Chewonarin T. The effect of Perilla frutescens leaf on 1, 2-dimethylhydrazine-induced initiation of colon carcinogenesis in rats. J. Food Biochem. 2018;42:e12493. doi: 10.1111/jfbc.12493. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

