Distinguishing externally from saccade-induced motion in visual cortex
- PMID: 36104560
- PMCID: PMC9534749
- DOI: 10.1038/s41586-022-05196-w
Distinguishing externally from saccade-induced motion in visual cortex
Erratum in
-
Publisher Correction: Distinguishing externally from saccade-induced motion in visual cortex.Nature. 2022 Nov;611(7934):E5. doi: 10.1038/s41586-022-05428-z. Nature. 2022. PMID: 36241716 Free PMC article. No abstract available.
Abstract
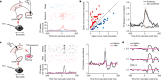
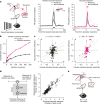
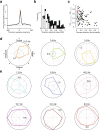
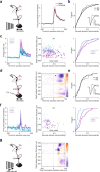
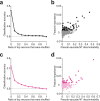
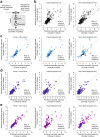
Distinguishing sensory stimuli caused by changes in the environment from those caused by an animal's own actions is a hallmark of sensory processing1. Saccades are rapid eye movements that shift the image on the retina. How visual systems differentiate motion of the image induced by saccades from actual motion in the environment is not fully understood2. Here we discovered that in mouse primary visual cortex (V1) the two types of motion evoke distinct activity patterns. This is because, during saccades, V1 combines the visual input with a strong non-visual input arriving from the thalamic pulvinar nucleus. The non-visual input triggers responses that are specific to the direction of the saccade and the visual input triggers responses that are specific to the direction of the shift of the stimulus on the retina, yet the preferred directions of these two responses are uncorrelated. Thus, the pulvinar input ensures differential V1 responses to external and self-generated motion. Integration of external sensory information with information about body movement may be a general mechanism for sensory cortices to distinguish between self-generated and external stimuli.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






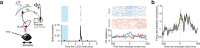


References
-
- Castet, E. Perception of intra-saccadic motion. in Dynamics of Visual Motion Processing: Neuronal, Behavioral, and Computational Approaches (eds Ilg, U. J. & Masson, G. S.) 213–238 (Springer US, 2010).
-
- Land MF. The evolution of gaze shifting eye movements. Curr. Top. Behav. Neurosci. 2019;41:3–11. - PubMed
-
- Land M. Eye movements in man and other animals. Vision Res. 2019;162:1–7. - PubMed

