Control of cell state transitions
- PMID: 36104561
- PMCID: PMC9644236
- DOI: 10.1038/s41586-022-05194-y
Control of cell state transitions
Abstract
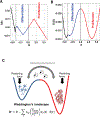
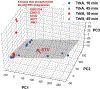
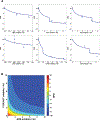
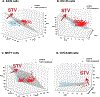
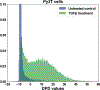
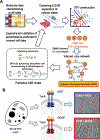
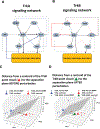
Understanding cell state transitions and purposefully controlling them is a longstanding challenge in biology. Here we present cell state transition assessment and regulation (cSTAR), an approach for mapping cell states, modelling transitions between them and predicting targeted interventions to convert cell fate decisions. cSTAR uses omics data as input, classifies cell states, and develops a workflow that transforms the input data into mechanistic models that identify a core signalling network, which controls cell fate transitions by influencing whole-cell networks. By integrating signalling and phenotypic data, cSTAR models how cells manoeuvre in Waddington's landscape1 and make decisions about which cell fate to adopt. Notably, cSTAR devises interventions to control the movement of cells in Waddington's landscape. Testing cSTAR in a cellular model of differentiation and proliferation shows a high correlation between quantitative predictions and experimental data. Applying cSTAR to different types of perturbation and omics datasets, including single-cell data, demonstrates its flexibility and scalability and provides new biological insights. The ability of cSTAR to identify targeted perturbations that interconvert cell fates will enable designer approaches for manipulating cellular development pathways and mechanistically underpinned therapeutic interventions.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures

















References
-
- Waddington CH Organisers and Genes by C. H. Waddington. (The University Press, 1940).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

