Pretreatment of the ROS Inhibitor Phenyl-N-tert-butylnitrone Alleviates Sleep Deprivation-Induced Hyperalgesia by Suppressing Microglia Activation and NLRP3 Inflammasome Activity in the Spinal Dorsal Cord
- PMID: 36104611
- PMCID: PMC9823061
- DOI: 10.1007/s11064-022-03751-5
Pretreatment of the ROS Inhibitor Phenyl-N-tert-butylnitrone Alleviates Sleep Deprivation-Induced Hyperalgesia by Suppressing Microglia Activation and NLRP3 Inflammasome Activity in the Spinal Dorsal Cord
Abstract
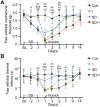
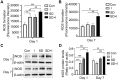
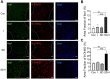
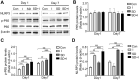
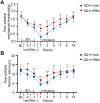
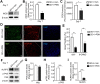
Sleep deprivation, a common perioperative period health problem, causes ocular discomfort and affects postsurgical pain. However, the mechanism of sleep deprivation-induced increased pain sensitivity is elusive. This study aims to explore the role of ROS in sleep deprivation (SD)-induced hyperalgesia and the underlying mechanism. A 48-h continuous SD was performed prior to the hind paw incision pain modeling in mice. We measured ROS levels, microglial activation, DNA damage and protein levels of iNOS, NLRP3, p-P65 and P65 in mouse spinal dorsal cord. The involvement of ROS in SD-induced prolongation of postsurgical pain was further confirmed by intrathecal injection of ROS inhibitor, phenyl-N-tert-butylnitrone (PBN). Pretreatment of 48-h SD in mice significantly prolonged postsurgical pain recovery, manifesting as lowered paw withdrawal mechanical threshold and paw withdrawal thermal latency. It caused ROS increase and upregulation of iNOS on both Day 1 and 7 in mouse spinal dorsal cord. In addition, upregulation of NLRP3 and p-P65, microglial activation and DNA damage were observed in mice pretreated with 48-h SD prior to the incision. Notably, intrathecal injection of PBN significantly reversed the harmful effects of SD on postsurgical pain recovery, hyperalgesia, microglial activation and DNA damage via the NF-κB signaling pathway. Collectively, ROS increase is responsible for SD-induced hyperalgesia through activating microglial, triggering DNA damage and enhancing NLRP3 inflammasome activity in the spinal dorsal cord.
Keywords: Chronic postsurgical pain; Microglia activation; NLRP3; Reactive oxygen species; Sleep deprivation.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
Similar articles
-
Remifentanil-induced inflammation in microglial cells: Activation of the PAK4-mediated NF-κB/NLRP3 pathway and onset of hyperalgesia.Brain Behav Immun. 2025 Jan;123:334-352. doi: 10.1016/j.bbi.2024.09.018. Epub 2024 Sep 24. Brain Behav Immun. 2025. PMID: 39322089
-
Spinal mitochondrial-derived ROS contributes to remifentanil-induced postoperative hyperalgesia via modulating NMDA receptor in rats.Neurosci Lett. 2016 Nov 10;634:79-86. doi: 10.1016/j.neulet.2016.09.016. Epub 2016 Sep 13. Neurosci Lett. 2016. PMID: 27637388
-
Activation of spinal PGC-1α regulates microglial polarization through a feedback loop between ROS-mediated mitochondrial dysfunction and the NLRP3 inflammasome in neuropathic pain.Brain Res Bull. 2025 Jul;227:111365. doi: 10.1016/j.brainresbull.2025.111365. Epub 2025 Apr 30. Brain Res Bull. 2025. PMID: 40316183
-
Blocking SphK/S1P/S1PR1 axis signaling pathway alleviates remifentanil-induced hyperalgesia in rats.Neurosci Lett. 2023 Mar 28;801:137131. doi: 10.1016/j.neulet.2023.137131. Epub 2023 Feb 15. Neurosci Lett. 2023. PMID: 36801239
-
The effects of recovery sleep on pain perception: A systematic review.Neurosci Biobehav Rev. 2020 Jun;113:408-425. doi: 10.1016/j.neubiorev.2020.03.028. Epub 2020 Apr 8. Neurosci Biobehav Rev. 2020. PMID: 32275917
Cited by
-
Temporal changes of spinal microglia in murine models of neuropathic pain: a scoping review.Front Immunol. 2024 Dec 6;15:1460072. doi: 10.3389/fimmu.2024.1460072. eCollection 2024. Front Immunol. 2024. PMID: 39735541 Free PMC article.
-
Dehydrocorydaline alleviates sleep deprivation-induced persistent postoperative pain in adolescent mice through inhibiting microglial P2Y12 receptor expression in the spinal cord.Mol Pain. 2023 Jan-Dec;19:17448069231216234. doi: 10.1177/17448069231216234. Mol Pain. 2023. PMID: 37940138 Free PMC article.
-
A Narrative Review of the Reciprocal Relationship Between Sleep Deprivation and Chronic Pain: The Role of Oxidative Stress.J Pain Res. 2024 May 20;17:1785-1792. doi: 10.2147/JPR.S455621. eCollection 2024. J Pain Res. 2024. PMID: 38799272 Free PMC article. Review.
-
Glia: the cellular glue that binds circadian rhythms and sleep.Sleep. 2025 Mar 11;48(3):zsae314. doi: 10.1093/sleep/zsae314. Sleep. 2025. PMID: 39812780 Free PMC article. Review.
-
Glucose competition between endothelial cells in the blood-spinal cord barrier and infiltrating regulatory T cells is linked to sleep restriction-induced hyperalgesia.BMC Med. 2024 May 7;22(1):189. doi: 10.1186/s12916-024-03413-z. BMC Med. 2024. PMID: 38715017 Free PMC article.
References
-
- Meersch M, Weiss R, Kullmar M, Bergmann L, Thompson A, Griep L, Kusmierz D, Buchholz A, Wolf A, Nowak H, Rahmel T, Adamzik M, Haaker JG, Goettker C, Gruendel M, Hemping-Bovenkerk A, Goebel U, Braumann J, Wisudanto I, Wenk M, Flores-Bergmann D, Bohmer A, Cleophas S, Hohn A, Houben A, Ellerkmann RK, Larmann J, Sander J, Weigand MA, Eick N, Ziemann S, Bormann E, Gerss J, Sessler DI, Wempe C, Massoth C, Zarbock A. Effect of intraoperative handovers of anesthesia care on mortality, readmission, or postoperative complications among adults: The HandiCAP Randomized Clinical Trial. JAMA. 2022;327:2403. doi: 10.1001/jama.2022.9451. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

