Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis
- PMID: 36104692
- PMCID: PMC9476391
- DOI: 10.1186/s12916-022-02500-3
Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis
Abstract
Background: Endometriosis is a common, complex disorder which is underrecognized and subject to prolonged delays in diagnosis. It is accompanied by significant changes in the eutopic endometrial lining.
Methods: We have undertaken the first single-cell RNA-sequencing (scRNA-Seq) comparison of endometrial tissues in freshly collected menstrual effluent (ME) from 33 subjects, including confirmed endometriosis patients (cases) and controls as well as symptomatic subjects (who have chronic symptoms suggestive of endometriosis but have not been diagnosed).
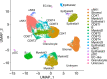
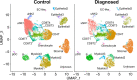
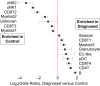
Results: We identify a unique subcluster of proliferating uterine natural killer (uNK) cells in ME-tissues from controls that is almost absent from endometriosis cases, along with a striking reduction of total uNK cells in the ME of cases (p < 10-16). In addition, an IGFBP1+ decidualized subset of endometrial stromal cells are abundant in the shed endometrium of controls when compared to cases (p < 10-16) confirming findings of compromised decidualization of cultured stromal cells from cases. By contrast, endometrial stromal cells from cases are enriched in cells expressing pro-inflammatory and senescent phenotypes. An enrichment of B cells in the cases (p = 5.8 × 10-6) raises the possibility that some may have chronic endometritis, a disorder which predisposes to endometriosis.
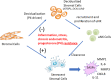
Conclusions: We propose that characterization of endometrial tissues in ME will provide an effective screening tool for identifying endometriosis in patients with chronic symptoms suggestive of this disorder. This constitutes a major advance, since delayed diagnosis for many years is a major clinical problem in the evaluation of these patients. Comprehensive analysis of ME is expected to lead to new diagnostic and therapeutic approaches to endometriosis and other associated reproductive disorders such as female infertility.
Keywords: Biomarkers; Decidualization; Endometriosis; Fibrosis; Inflammation; Menstrual blood; Menstrual effluent; Senescence; Single-cell RNA sequencing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–154. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

