Captopril alleviates epilepsy and cognitive impairment by attenuation of C3-mediated inflammation and synaptic phagocytosis
- PMID: 36104755
- PMCID: PMC9476304
- DOI: 10.1186/s12974-022-02587-8
Captopril alleviates epilepsy and cognitive impairment by attenuation of C3-mediated inflammation and synaptic phagocytosis
Abstract
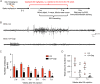
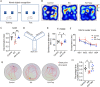
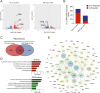
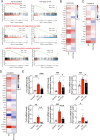
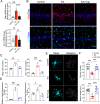
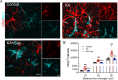
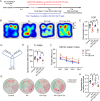
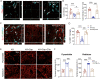
Evidence from experimental and clinical studies implicates immuno-inflammatory responses as playing an important role in epilepsy-induced brain injury. Captopril, an angiotensin-converting enzyme inhibitor (ACEi), has previously been shown to suppress immuno-inflammatory responses in a variety of neurological diseases. However, the therapeutic potential of captopril on epilepsy remains unclear. In the present study, Sprague Dawley (SD) rats were intraperitoneally subjected to kainic acid (KA) to establish a status epilepticus. Captopril (50 mg/kg, i.p.) was administered daily following the KA administration from day 3 to 49. We found that captopril efficiently suppressed the KA-induced epilepsy, as measured by electroencephalography. Moreover, captopril ameliorated the epilepsy-induced cognitive deficits, with improved performance in the Morris water maze, Y-maze and novel objective test. RNA sequencing (RNA-seq) analysis indicated that captopril reversed a wide range of epilepsy-related biological processes, particularly the glial activation, complement system-mediated phagocytosis and the production of inflammatory factors. Interestingly, captopril suppressed the epilepsy-induced activation and abnormal contact between astrocytes and microglia. Immunohistochemical experiments demonstrated that captopril attenuated microglia-dependent synaptic remodeling presumably through C3-C3ar-mediated phagocytosis in the hippocampus. Finally, the above effects of captopril were partially blocked by an intranasal application of recombinant C3a (1.3 μg/kg/day). Our findings demonstrated that captopril reduced the occurrence of epilepsy and cognitive impairment by attenuation of inflammation and C3-mediated synaptic phagocytosis. This approach can easily be adapted to long-term efficacy and safety in clinical practice.
Keywords: Captopril; Cognitive deficits; Complement 3; Epilepsy; Glial activation; Synaptic phagocytosis.
© 2022. The Author(s).
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



Comment in
-
Who Dunnit? Angiotensin, Inflammation, or Complement: Unresolved.Epilepsy Curr. 2023 Jan 18;23(2):133-135. doi: 10.1177/15357597221150057. eCollection 2023 Mar-Apr. Epilepsy Curr. 2023. PMID: 37122407 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

