Quantitative impact of pre-analytical process on plasma uracil when testing for dihydropyrimidine dehydrogenase deficiency
- PMID: 36104927
- PMCID: PMC10092089
- DOI: 10.1111/bcp.15536
Quantitative impact of pre-analytical process on plasma uracil when testing for dihydropyrimidine dehydrogenase deficiency
Abstract
Aims: Determining dihydropyrimidine dehydrogenase (DPD) activity by measuring patient's uracil (U) plasma concentration is mandatory before fluoropyrimidine (FP) administration in France. In this study, we aimed to refine the pre-analytical recommendations for determining U and dihydrouracil (UH2 ) concentrations, as they are essential in reliable DPD-deficiency testing.
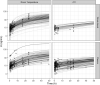
Methods: U and UH2 concentrations were collected from 14 hospital laboratories. Stability in whole blood and plasma after centrifugation, the type of anticoagulant and long-term plasma storage were evaluated. The variation induced by time and temperature was calculated and compared to an acceptability range of ±20%. Inter-occasion variability (IOV) of U and UH2 was assessed in 573 patients double sampled for DPD-deficiency testing.
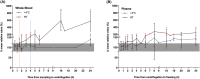
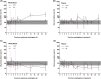
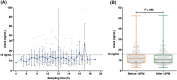
Results: Storage of blood samples before centrifugation at room temperature (RT) should not exceed 1 h, whereas cold (+4°C) storage maintains the stability of uracil after 5 hours. For patients correctly double sampled, IOV of U reached 22.4% for U (SD = 17.9%, range = 0-99%). Notably, 17% of them were assigned with a different phenotype (normal or DPD-deficient) based on the analysis of their two samples. For those having at least one non-compliant sample, this percentage increased up to 33.8%. The moment of blood collection did not affect the DPD phenotyping result.
Conclusion: Caution should be taken when interpreting U concentrations if the time before centrifugation exceeds 1 hour at RT, since it rises significantly afterwards. Not respecting the pre-analytical conditions for DPD phenotyping increases the risk of DPD status misclassification.
Keywords: dihydropyrimidine dehydrogenase; dihydrouracil; intra-individual variability; pre-analytical practices; uracil.
© 2022 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- HAS‐Santé . Dihydropyrimidine dehydrogenase deficiency testing to prevent fluoropyrimidines‐associated severe toxicities. 2018. https://www.has-sante.fr/jcms/c_2891090/en/screening-for-dihydropyrimidi.... Accessed September 20, 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

