GNB3 c.825c>T polymorphism influences T-cell but not antibody response following vaccination with the mRNA-1273 vaccine
- PMID: 36105097
- PMCID: PMC9465595
- DOI: 10.3389/fgene.2022.932043
GNB3 c.825c>T polymorphism influences T-cell but not antibody response following vaccination with the mRNA-1273 vaccine
Abstract
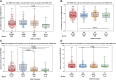
Background: Immune responses following vaccination against COVID-19 with different vaccines and the waning of immunity vary within the population. Genetic host factors are likely to contribute to this variability. However, to the best of our knowledge, no study on G protein polymorphisms and vaccination responses against COVID-19 has been published so far. Methods: Antibodies against the SARS-CoV-2 spike protein and T-cell responses against a peptide pool of SARS-CoV-2 S1 proteins were measured 1 and 6 months after the second vaccination with mRNA-1273 in the main study group of 204 participants. Additionally, antibodies against the SARS-CoV-2 spike protein were measured in a group of 597 participants 1 month after the second vaccination with mRNA-1273. Genotypes of GNB3 c.825C>T were determined in all participants. Results: The median antibody titer against the SARS-CoV-2 spike protein and median values of spots increment in the SARS-CoV-2 IFN-γ ELISpot assay against the S1-peptide pool were significantly decreased from months 1 to 6 (p < 0.0001). Genotypes of GNB3 c.825C>T had no influence on the humoral immune response. At month 1, CC genotype carriers had significantly increased T-cell responses compared to CT (p = 0.005) or TT (p = 0.02) genotypes. CC genotype carriers had an almost 6-fold increased probability compared to TT genotype carriers and an almost 3-fold increased probability compared to T-allele carriers to mount a SARS-CoV-2-specific T-cell response above the median value. Conclusion: CC genotype carriers of the GNB3 c.825C>T polymorphism have an increased T-cell immune response to SARS-CoV-2, which may indicate better T-cell-mediated protection against COVID-19 after vaccination with mRNA-1273.
Keywords: COVID-19; ELISpot; GNB3 c.825C>T; SARS-CoV-2; SARS-CoV-2 spike antibody titer; antigen-specific T-cell response; mRNA-1273.
Copyright © 2022 Čiučiulkaitė, Möhlendick, Thümmler, Fisenkci, Elsner, Dittmer, Siffert and Lindemann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Gallagher K. M. E., Leick M. B., Larson R. C., Berger T. R., Katsis K., Yam J. Y., et al. (2022). Differential T-cell immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in mRNA-1273– and BNT162b2-vaccinated individuals. Clin. Infect. Dis., ciac201. 10.1093/cid/ciac201 - DOI - PMC - PubMed
-
- Geisen U. M., Berner D. K., Tran F., Sumbul M., Vullriede L., Ciripoi M., et al. (2021). Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 80 (10), 1306–1311. 10.1136/annrheumdis-2021-220272 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

